How Subtle Changes Can Make a Difference: Reproducibility in Complex Supramolecular Systems
- PMID: 36062929
- PMCID: PMC9825988
- DOI: 10.1002/anie.202206738
How Subtle Changes Can Make a Difference: Reproducibility in Complex Supramolecular Systems
Abstract
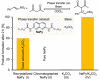
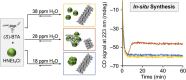
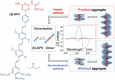
The desire to construct complex molecular systems is driven by the need for technological (r)evolution and our intrinsic curiosity to comprehend the origin of life. Supramolecular chemists tackle this challenge by combining covalent and noncovalent reactions leading to multicomponent systems with emerging complexity. However, this synthetic strategy often coincides with difficult preparation protocols and a narrow window of suitable conditions. Here, we report on unsuspected observations of our group that highlight the impact of subtle "irregularities" on supramolecular systems. Based on the effects of pathway complexity, minute amounts of water in organic solvents or small impurities in the supramolecular building block, we discuss potential pitfalls in the study of complex systems. This article is intended to draw attention to often overlooked details and to initiate an open discussion on the importance of reporting experimental details to increase reproducibility in supramolecular chemistry.
Keywords: Complexity; Reproducibility; Solvent Effects; Supramolecular Systems.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lutz J.-F., Lehn J.-M., Meijer E. W., Matyjaszewski K., Nat. Rev. Mater. 2016, 1, 16024.
-
- Feringa B. L., Angew. Chem. Int. Ed. 2017, 56, 11060; - PubMed
- Angew. Chem. 2017, 129, 11206.
-
- Stoddart J. F., Angew. Chem. Int. Ed. 2017, 56, 11094; - PubMed
- Angew. Chem. 2017, 129, 11244.
-
- Sauvage J. P., Angew. Chem. Int. Ed. 2017, 56, 11080; - PubMed
- Angew. Chem. 2017, 129, 11228.
-
- Vantomme G., Meijer E. W., Science 2019, 363, 1396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

