What is reproductive isolation?
- PMID: 36063156
- PMCID: PMC9542822
- DOI: 10.1111/jeb.14005
What is reproductive isolation?
Abstract
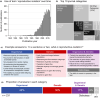
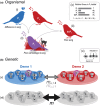
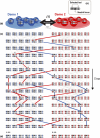
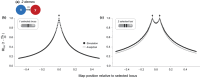
Reproductive isolation (RI) is a core concept in evolutionary biology. It has been the central focus of speciation research since the modern synthesis and is the basis by which biological species are defined. Despite this, the term is used in seemingly different ways, and attempts to quantify RI have used very different approaches. After showing that the field lacks a clear definition of the term, we attempt to clarify key issues, including what RI is, how it can be quantified in principle, and how it can be measured in practice. Following other definitions with a genetic focus, we propose that RI is a quantitative measure of the effect that genetic differences between populations have on gene flow. Specifically, RI compares the flow of neutral alleles in the presence of these genetic differences to the flow without any such differences. RI is thus greater than zero when genetic differences between populations reduce the flow of neutral alleles between populations. We show how RI can be quantified in a range of scenarios. A key conclusion is that RI depends strongly on circumstances-including the spatial, temporal and genomic context-making it difficult to compare across systems. After reviewing methods for estimating RI from data, we conclude that it is difficult to measure in practice. We discuss our findings in light of the goals of speciation research and encourage the use of methods for estimating RI that integrate organismal and genetic approaches.
Keywords: adaptation; genomics; natural selection; population genetics; speciation; theory.
© 2022 The Authors. Journal of Evolutionary Biology published by John Wiley & Sons Ltd on behalf of European Society for Evolutionary Biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
The language of isolation: a commentary on Westram et al., 2022.J Evol Biol. 2022 Sep;35(9):1195-1199. doi: 10.1111/jeb.14029. J Evol Biol. 2022. PMID: 36063154 Free PMC article. No abstract available.
-
Using neutral loci to quantify reproductive isolation and speciation: a commentary on Westram et al., 2022.J Evol Biol. 2022 Sep;35(9):1169-1174. doi: 10.1111/jeb.14057. J Evol Biol. 2022. PMID: 36063155 No abstract available.
-
Forty-two, and other precise answers to difficult questions: a commentary on Westram et al., 2022.J Evol Biol. 2022 Sep;35(9):1183-1187. doi: 10.1111/jeb.14037. J Evol Biol. 2022. PMID: 36063157 No abstract available.
-
Epigenetics and reproductive isolation: a commentary on Westram et al., 2022.J Evol Biol. 2022 Sep;35(9):1188-1194. doi: 10.1111/jeb.14033. J Evol Biol. 2022. PMID: 36063158 Free PMC article. No abstract available.
-
What is in a definition? Reflections on a framework for understanding reproductive isolation: a commentary on Westram et al., 2022.J Evol Biol. 2022 Sep;35(9):1165-1168. doi: 10.1111/jeb.14072. J Evol Biol. 2022. PMID: 36063159 No abstract available.
-
Reproductive isolation, speciation, and the value of disagreement: A reply to the commentaries on 'What is reproductive isolation?'.J Evol Biol. 2022 Sep;35(9):1200-1205. doi: 10.1111/jeb.14082. J Evol Biol. 2022. PMID: 36063160 Free PMC article. No abstract available.
-
Reproductive isolation is a heuristic, not a measure: a commentary on Westram et al., 2022.J Evol Biol. 2022 Sep;35(9):1175-1182. doi: 10.1111/jeb.14052. J Evol Biol. 2022. PMID: 36063161
References
-
- Barton, N. H. (1980). The hybrid sink effect. Heredity, 44, 277–278. 10.1038/hdy.1980.23 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

