Biofabrication of Human Skin with Its Appendages
- PMID: 36063498
- PMCID: PMC11469047
- DOI: 10.1002/adhm.202201626
Biofabrication of Human Skin with Its Appendages
Abstract
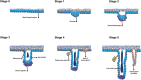
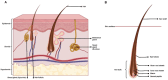
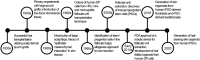
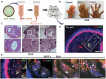
Much effort has been made to generate human skin organ in the laboratory. Yet, the current models are limited due to the lack of many critical biological and structural features of the skin. Importantly, these in vitro models lack appendages and fail to recapitulate the whole human skin construction. Thus, engineering a human skin with the capacity to generate all components, including appendages, is a major challenge. This review intends to provide an update on the recent efforts underway to regenerate appendage-bearing skin organs based on scaffold-free and scaffold-based bioengineering approaches. Although the mouse skin equivalents containing hair follicles, sebaceous glands, and sweat glands have been established in vitro, there has been limited success in humans. A combination of biofabricated matrices and cell aggregates, such as organoids, can pave the way for generating skin substitutes with human-like biological, structural, and physical features. Accordingly, the formation of human skin organoids and reconstruction of vascularized skin equipped with immune cells prompt calls for more scientific research. The generation of appendage-bearing skin substitutes can be applied in practice for wound healing, hair restoration, and scar treatment.
Keywords: 3D culture; bioengineering; regenerative medicine; tissue engineering; wounds.
© 2022 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

