Nrf2 transcriptional upregulation of IDH2 to tune mitochondrial dynamics and rescue angiogenic function of diabetic EPCs
- PMID: 36063728
- PMCID: PMC9463384
- DOI: 10.1016/j.redox.2022.102449
Nrf2 transcriptional upregulation of IDH2 to tune mitochondrial dynamics and rescue angiogenic function of diabetic EPCs
Abstract
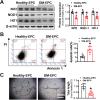
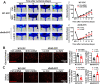
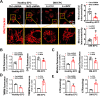
Endothelial progenitor cells (EPCs) are reduced in number and impaired in function in diabetic patients. Whether and how Nrf2 regulates the function of diabetic EPCs remains unclear. In this study, we found that the expression of Nrf2 and its downstream genes were decreased in EPCs from both diabetic patients and db/db mice. Survival ability and angiogenic function of EPCs from diabetic patients and db/db mice also were impaired. Gain- and loss-of-function studies, respectively, showed that knockdown of Nrf2 increased apoptosis and impaired tube formation in EPCs from healthy donors and wild-type mice, while Nrf2 overexpression decreased apoptosis and rescued tube formation in EPCs from diabetic patients and db/db mice. Additionally, proangiogenic function of Nrf2-manipulated mouse EPCs was validated in db/db mice with hind limb ischemia. Mechanistic studies demonstrated that diabetes induced mitochondrial fragmentation and dysfunction of EPCs by dysregulating the abundance of proteins controlling mitochondrial dynamics; upregulating Nrf2 expression attenuated diabetes-induced mitochondrial fragmentation and dysfunction and rectified the abundance of proteins controlling mitochondrial dynamics. Further RNA-sequencing analysis demonstrated that Nrf2 specifically upregulated the transcription of isocitrate dehydrogenase 2 (IDH2), a key enzyme regulating tricarboxylic acid cycle and mitochondrial function. Overexpression of IDH2 rectified Nrf2 knockdown- or diabetes-induced mitochondrial fragmentation and EPC dysfunction. In a therapeutic approach, supplementation of an Nrf2 activator sulforaphane enhanced angiogenesis and blood perfusion recovery in db/db mice with hind limb ischemia. Collectively, these findings indicate that Nrf2 is a potential therapeutic target for improving diabetic EPC function. Thus, elevating Nrf2 expression enhances EPC resistance to diabetes-induced oxidative damage and improves therapeutic efficacy of EPCs in treating diabetic limb ischemia likely via transcriptional upregulating IDH2 expression and improving mitochondrial function of diabetic EPCs.
Keywords: Angiogenesis; Diabetes; Endothelial progenitor cell; Hind limb ischemia; Nuclear factor erythroid 2-related factor 2.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest No potential conflicts of interest relevant to this article were reported.
Figures





References
-
- Dai X., Yan X., Zeng J., Chen J., Wang Y., Chen J., Li Y., Barati M.T., Wintergerst K.A., Pan K., Nystoriak M.A., Conklin D.J., Rokosh G., Epstein P.N., Li X., Tan Y. Elevating cxcr7 improves angiogenic function of epcs via akt/gsk-3beta/fyn-mediated nrf2 activation in diabetic limb ischemia. Circ. Res. 2017;120:e7–e23. - PMC - PubMed
-
- Butler J.M., Nolan D.J., Vertes E.L., Varnum-Finney B., Kobayashi H., Hooper A.T., Seandel M., Shido K., White I.A., Kobayashi M., Witte L., May C., Shawber C., Kimura Y., Kitajewski J., Rosenwaks Z., Bernstein I.D., Rafii S. Endothelial cells are essential for the self-renewal and repopulation of notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

