Neutrophil extracellular traps contribute to liver damage and increase defective low-density neutrophils in alcohol-associated hepatitis
- PMID: 36063965
- PMCID: PMC11910133
- DOI: 10.1016/j.jhep.2022.08.029
Neutrophil extracellular traps contribute to liver damage and increase defective low-density neutrophils in alcohol-associated hepatitis
Abstract
Background & aims: In alcohol-associated hepatitis (AH), inflammation and neutrophil counts correlate with poor clinical outcomes. Here, we investigated how neutrophils contribute to liver damage in AH.
Methods: We isolated blood neutrophils from individuals with AH to examine neutrophil extracellular traps (NETs) and performed RNA sequencing to explore their unique characteristics.
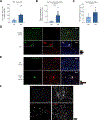
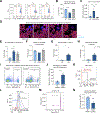
Results: We observed a significant increase in NET production in AH. We also observed a unique low-density neutrophil (LDN) population in individuals with AH and alcohol-fed mice that was not present in healthy controls. Transcriptome analysis of peripheral LDNs and high-density neutrophils (HDNs) from individuals with AH revealed that LDNs exhibit a functionally exhausted phenotype, while HDNs are activated. Indeed, AH HDNs exhibited increased resting reactive oxygen species (ROS) production and produced more ROS upon lipopolysaccharide stimulation than control HDNs, whereas AH LDNs failed to respond to lipopolysaccharide. We show that LDNs are generated from HDNs after alcohol-induced NET release in vitro, and this LDN subset has decreased functionality, including reduced phagocytic capacity. Moreover, LDNs showed reduced homing capacity and clearance by macrophage efferocytosis; therefore, dysfunctional neutrophils could remain in the circulation and liver. Depletion of both HDNs and LDNs in vivo prevented alcohol-induced NET production and liver damage in mice. Granulocyte-colony stimulating factor treatment also ameliorated alcohol-induced liver injury in mice.
Conclusion: Neutrophils contribute to liver damage through increased NET formation which increases defective LDNs in AH. Alcohol induces phenotypic changes in neutrophils; HDNs are activated whereas LDNs are defective. Our findings provide mechanistic insights that could guide the development of therapeutic interventions for AH.
Impact and implications: In this study we discovered heterogeneity of neutrophils in alcohol-associated hepatitis, including high-density and low-density neutrophils that show hyper-activated or exhausted transcriptomic profiles, respectively. We found that alcohol induces neutrophil extracellular trap (NET) formation, which contributes to liver damage. NET release by high-density neutrophils resulted in low-density neutrophils that reside in the liver and escape clean-up by macrophages. Our findings help to understand the opposing neutrophil phenotypes observed in individuals with alcohol-associated hepatitis and provide mechanistic insights that could guide therapeutic strategies targeting neutrophils.
Keywords: Low-density neutrophils (LDNs); Neutrophil extracellular traps (NETs); alcoholic hepatitis.
Copyright © 2022 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
G.S. reports being a paid consult for Durect Corporation, Cyta Therapeutics, Evive, Glympse Bio, Terra Firma, Pandion Therapeutics, Surrozen, Ventyx, Merck, Novartis, Pfizer and Zomagen. I.S.V reports consulting for Mosaic Research Management.
Please refer to the accompanying ICMJE disclosure forms for further details.
Figures







References
-
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009;360(26):2758–2769. - PubMed
-
- Singal AK, Shah VH. Current trials and novel therapeutic targets for alcoholic hepatitis. J Hepatol 2019;70(2):305–313. - PubMed
-
- Forrest EH, Storey N, Sinha R, Atkinson SR, Vergis N, Richardson P, et al. Baseline neutrophil-to-lymphocyte ratio predicts response to corticosteroids and is associated with infection and renal dysfunction in alcoholic hepatitis. Aliment Pharmacol Ther 2019;50(4):442–453. - PubMed
-
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13(3):159–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

