Aging exacerbates the brain inflammatory micro-environment contributing to α-synuclein pathology and functional deficits in a mouse model of DLB/PD
- PMID: 36064424
- PMCID: PMC9447339
- DOI: 10.1186/s13024-022-00564-6
Aging exacerbates the brain inflammatory micro-environment contributing to α-synuclein pathology and functional deficits in a mouse model of DLB/PD
Retraction in
-
Retraction Note: Aging exacerbates the brain inflammatory micro-environment contributing to α-synuclein pathology and functional deficits in a mouse model of DLB/PD.Mol Neurodegener. 2024 Oct 16;19(1):74. doi: 10.1186/s13024-024-00762-4. Mol Neurodegener. 2024. PMID: 39415232 Free PMC article. No abstract available.
Abstract
Background: Although ɑ-synuclein (ɑ-syn) spreading in age-related neurodegenerative diseases such as Parkinson's disease (PD) and Dementia with Lewy bodies (DLB) has been extensively investigated, the role of aging in the manifestation of disease remains unclear.
Methods: We explored the role of aging and inflammation in the pathogenesis of synucleinopathies in a mouse model of DLB/PD initiated by intrastriatal injection of ɑ-syn preformed fibrils (pff).
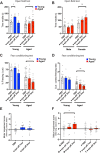
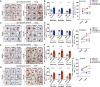
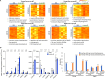
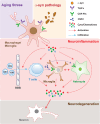
Results: We found that aged mice showed more extensive accumulation of ɑ-syn in selected brain regions and behavioral deficits that were associated with greater infiltration of T cells and microgliosis. Microglial inflammatory gene expression induced by ɑ-syn-pff injection in young mice had hallmarks of aged microglia, indicating that enhanced age-associated pathologies may result from inflammatory synergy between aging and the effects of ɑ-syn aggregation. Based on the transcriptomics analysis projected from Ingenuity Pathway Analysis, we found a network that included colony stimulating factor 2 (CSF2), LPS related genes, TNFɑ and poly rl:rC-RNA as common regulators.
Conclusions: We propose that aging related inflammation (eg: CSF2) influences outcomes of pathological spreading of ɑ-syn and suggest that targeting neuro-immune responses might be important in developing treatments for DLB/PD.
Keywords: Aging; Dementia with Lewy bodies; Inflammation; Microglia; Neurodegeneration; Parkinson’s disease; Preformed fibrils; RNA-seq; T cell infiltration; ɑ-synuclein.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–81. - PubMed
-
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. - PubMed
-
- Kotzbauer PT, Trojanowsk JQ, Lee VM. Lewy body pathology in Alzheimer’s disease. J Mol Neurosci. 2001;17:225–32. - PubMed
-
- Alafuzoff I, Hartikainen P. Alpha-synucleinopathies. Handb Clin Neurol. 2017;145:339–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

