Synovial membrane-derived mesenchymal progenitor cells from osteoarthritic joints in dogs possess lower chondrogenic-, and higher osteogenic capacity compared to normal joints
- PMID: 36064441
- PMCID: PMC9446738
- DOI: 10.1186/s13287-022-03144-z
Synovial membrane-derived mesenchymal progenitor cells from osteoarthritic joints in dogs possess lower chondrogenic-, and higher osteogenic capacity compared to normal joints
Abstract
Background: Synovial membrane-derived mesenchymal progenitor cells (SM-MPCs) are a promising candidate for the cell-based treatment of osteoarthritis (OA) considering their in vitro and in vivo capacity for cartilage repair. However, the OA environment may adversely impact their regenerative capacity. There are no studies for canine (c)SM-MPCs that compare normal to OA SM-MPCs, even though dogs are considered a relevant animal model for OA. Therefore, this study compared cSM-MPCs from normal and OA synovial membrane tissue to elucidate the effect of the OA environment on MPC numbers, indicated by CD marker profile and colony-forming unit (CFU) capacity, and the impact of the OA niche on tri-lineage differentiation.
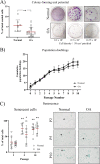
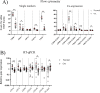
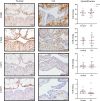
Methods: Normal and OA synovial membrane were collected from the knee joints of healthy dogs and dogs with rupture of the cruciate ligaments. The synovium was assessed by histopathological OARSI scoring and by RT-qPCR for inflammation/synovitis-related markers. The presence of cSM-MPCs in the native tissue was further characterized with flow cytometry, RT-qPCR, and immunohistochemistry, using the MPC markers; CD90, CD73, CD44, CD271, and CD34. Furthermore, cells isolated upon enzymatic digestion were characterized by CFU capacity, and a population doublings assay. cSM-MPCs were selected based on plastic adherence, expanded to passage 2, and evaluated for the expression of MPC-related surface markers and tri-lineage differentiation capacity.
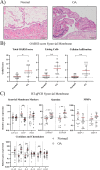
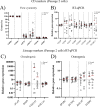
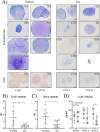
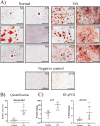
Results: Synovial tissue collected from the OA joints had a significantly higher OARSI score compared to normal joints, and significantly upregulated inflammation/synovitis markers S100A8/9, IL6, IL8, and CCL2. Both normal and OA synovial membrane contained cells displaying MPC properties, including a fibroblast-like morphology, CFU capacity, and maintained MPC marker expression over time during expansion. However, OA cSM-MPCs were unable to differentiate towards the chondrogenic lineage and had low adipogenic capacity in contrast to normal cSM-MPCs, whereas they possessed a higher osteogenic capacity. Furthermore, the OA synovial membrane contained significantly lower percentages of CD90+, CD44+, CD34+, and CD271+ cells.
Conclusions: The OA environment had adverse effects on the regenerative potential of cSM-MPCs, corroborated by decreased CFU, population doubling, and chondrogenic capacity compared to normal cSM-MPCs. OA cSM-MPCs may be a less optimal candidate for the cell-based treatment of OA than normal cSM-MPCs.
Keywords: CD271; CD34; Flow cytometry; Immunohistochemistry; Mesenchymal progenitor cells; Synovial membrane; Tri-lineage differentiation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S, et al. No identical “Mesenchymal Stem Cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Rep. 2016;6:897–913. doi: 10.1016/J.STEMCR.2016.05.011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

