Topical and intravenous administration of human umbilical cord mesenchymal stem cells in patients with diabetic foot ulcer and peripheral arterial disease: a phase I pilot study with a 3-year follow-up
- PMID: 36064461
- PMCID: PMC9446755
- DOI: 10.1186/s13287-022-03143-0
Topical and intravenous administration of human umbilical cord mesenchymal stem cells in patients with diabetic foot ulcer and peripheral arterial disease: a phase I pilot study with a 3-year follow-up
Abstract
Background: Diabetic foot ulcer (DFU) is a serious chronic complication of diabetes mellitus that contributes to 85% of nontraumatic lower extremity amputations in diabetic patients. Preliminary clinical benefits have been shown in treatments based on mesenchymal stem cells for patients with DFU or peripheral arterial disease (PAD). However, the long-term safety and benefits are unclear for patients with both DFU and PAD who are not amenable to surgical revascularization.
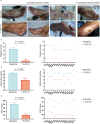
Methods: In this phase I pilot study, 14 patients with PAD and incurable DFU were enrolled to assess the safety and efficacy of human umbilical cord mesenchymal stem cell (hUC-MSC) administration based on conservative treatments. All patients received topical and intravenous administrations of hUC-MSCs at a dosage of 2 × 105 cells/kg with an upper limit of 1 × 107 cells for each dose. The adverse events during treatment and follow-up were documented for safety assessments. The therapeutic efficacy was assessed by ulcer healing status, recurrence rate, and 3-year amputation-free rate in the follow-up phase.
Results: The safety profiles were favorable. Only 2 cases of transient fever were observed within 3 days after transfusion and considered possibly related to hUC-MSC administration intravenously. Ulcer disclosure was achieved for more than 95% of the lesion area for all patients within 1.5 months after treatment. The symptoms of chronic limb ischaemia were alleviated along with a decrease in Wagner scores, Rutherford grades, and visual analogue scale scores. No direct evidence was observed to indicate the alleviation of the obstruction in the main vessels of target limbs based on computed tomography angiography. The duration of rehospitalization for DFU was 2.0 ± 0.6 years. All of the patients survived without amputation due to the recurrence of DFU within 3 years after treatments.
Conclusions: Based on the current pilot study, the preliminary clinical benefits of hUC-MSCs on DFU healing were shown, including good tolerance, a shortened healing time to 1.5 months and a favorable 3-year amputation-free survival rate. The clinical evidence in the current study suggested a further phase I/II study with a larger patient population and a more rigorous design to explore the efficacy and mechanism of hUC-MSCs on DFU healing.
Trial registration: The current study was registered retrospectively on 22 Jan 2022 with the Chinese Clinical Trial Registry (ChiCTR2200055885), http://www.chictr.org.cn/showproj.aspx?proj=135888.
Keywords: Diabetes complications; Diabetic foot ulcer; Human umbilical cord mesenchymal stem cells; Peripheral arterial disease.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Margolis D, Malay D, Hoffstad O, Leonard C, MaCurdy T, de Nava K, et al. Incidence of diabetic foot ulcer and lower extremity amputation among Medicare beneficiaries, 2006 to 2008: Data Points #2. In: Data Points Publication Series [Internet] Rockville (MD): Agency for Healthcare Research and Quality (US). 2011. - PubMed
-
- Hinchliffe RJ, Forsythe RO, Apelqvist J, Boyko EJ, Fitridge R, Hong JP, et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update) Diabetes Metab Res Rev. 2020;36(Suppl 1):e3276. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

