A Cryptochrome adopts distinct moon- and sunlight states and functions as sun- versus moonlight interpreter in monthly oscillator entrainment
- PMID: 36064778
- PMCID: PMC9445029
- DOI: 10.1038/s41467-022-32562-z
A Cryptochrome adopts distinct moon- and sunlight states and functions as sun- versus moonlight interpreter in monthly oscillator entrainment
Abstract
The moon's monthly cycle synchronizes reproduction in countless marine organisms. The mass-spawning bristle worm Platynereis dumerilii uses an endogenous monthly oscillator set by full moon to phase reproduction to specific days. But how do organisms recognize specific moon phases? We uncover that the light receptor L-Cryptochrome (L-Cry) discriminates between different moonlight durations, as well as between sun- and moonlight. A biochemical characterization of purified L-Cry protein, exposed to naturalistic sun- or moonlight, reveals the formation of distinct sun- and moonlight states characterized by different photoreduction- and recovery kinetics of L-Cry's co-factor Flavin Adenine Dinucleotide. In Platynereis, L-Cry's sun- versus moonlight states correlate with distinct subcellular localizations, indicating different signaling. In contrast, r-Opsin1, the most abundant ocular opsin, is not required for monthly oscillator entrainment. Our work reveals a photo-ecological concept for natural light interpretation involving a "valence interpreter" that provides entraining photoreceptor(s) with light source and moon phase information.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
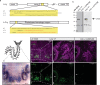
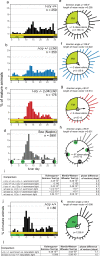





References
-
- Fox HM. Lunar periodicity in Reproduction. Proc. R. Soc. Lond. 1924;95:523–550.
-
- Numata, H. & Helm, B.Annual, Lunar, and Tidal Clocks: Patterns and Mechanisms of Nature’s Enigmatic Rhythms (Springer, 2014).
-
- Korringa P. Relations between the moon and periodicity in the breeding of marine animals. Ecol. Monogr. 1947;17:347–381. doi: 10.2307/1948665. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

