Fabrication of nanostructured lipid carriers ocugel for enhancing Loratadine used in treatment of COVID-19 related symptoms: statistical optimization, in-vitro, ex-vivo, and in-vivo studies evaluation
- PMID: 36065090
- PMCID: PMC9448409
- DOI: 10.1080/10717544.2022.2115164
Fabrication of nanostructured lipid carriers ocugel for enhancing Loratadine used in treatment of COVID-19 related symptoms: statistical optimization, in-vitro, ex-vivo, and in-vivo studies evaluation
Abstract
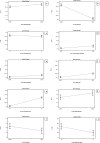
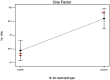
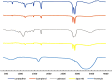
Loratadine (LORA), is a topical antihistamine utilized in the treatment of ocular symptoms of COVID-19. The study aimed to develop a Loratadine Nanostructured Lipid Carriers Ocugel (LORA-NLCs Ocugel), enhance its solubility, trans-corneal penetrability, and bioavailability. full-factorial design was established with 24 trials to investigate the impact of several variables upon NLCs properties. LORA-NLCs were fabricated by using hot melt emulsification combined with high-speed stirring and ultrasonication methods. All obtained formulae were assessed in terms of percent of entrapment efficiency (EE%), size of the particle (PS), zeta potential (ZP), as well as in-vitro release. Via using Design Expert® software the optimum formula was selected, characterized using FTIR, Raman spectroscopy, and stability studies. Gel-based of optimized LORA-NLCs was prepared using 4% HPMC k100m which was further evaluated in terms of physicochemical properties, Ex-vivo, and In-vivo studies. The optimized LORA-NLCs, comprising Compritol 888 ATO®, Labrasol®, and Span® 60 showed EE% of 95.78 ± 0.67%, PS of 156.11 ± 0.54 nm, ZP of -40.10 ± 0.55 Mv, and Qh6% of 99.67 ± 1.09%, respectively. Additionally, it illustrated a spherical morphology and compatibility of LORA with other excipients. Consequently, gel-based on optimized LORA-NLCs showed pH (7.11 ± 0.52), drug content (98.62%± 1.31%), viscosity 2736 cp, and Q12% (90.49 ± 1.32%). LORA-NLCs and LORA-NLCs Ocugel exhibited higher ex-vivo trans-corneal penetrability compared with the aqueous drug dispersion. Confocal laser scanning showed valuable penetration of fluoro-labeled optimized formula and LORA-NLCs Ocugel through corneal. The optimized formula was subjected to an ocular irritation test (Draize Test) that showed the absence of any signs of inflammation in rabbits, and histological analysis showed no effect or damage to rabbit eyeballs. Cmax and the AUC0-24 were higher in LORA-NLCs Ocugel compared with pure Lora dispersion-loaded gel The research findings confirmed that NLCs could enhance solubility, trans-corneal penetrability, and the bioavailability of LORA.
Keywords: Draize test; Loratadine (LORA); confocal laser scanning microscopy (CLSM); nanostructured lipid carriers; pharmacokinetic studies; reducing of COVID-19 ocular symptoms.
Conflict of interest statement
The authors report no conflict of interest.
Figures





References
-
- Abdel-Aziz RT, Aly UF, Mady FM. (2021). Enhanced skin delivery of propranolol HCl using nonionic surfactant-based vesicles for topical treatment of infantile hemangioma. J Drug Deliv Sci Technol 61:102235.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
