Salivary and serum IgA and IgG responses to SARS-CoV-2-spike protein following SARS-CoV-2 infection and after immunization with COVID-19 vaccines
- PMID: 36065108
- PMCID: PMC9465644
- DOI: 10.2500/aap.2022.43.220045
Salivary and serum IgA and IgG responses to SARS-CoV-2-spike protein following SARS-CoV-2 infection and after immunization with COVID-19 vaccines
Abstract
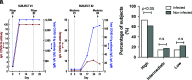
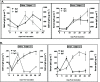
Background: Secretory immunoglobulin A (sIgA) plays an important role in antiviral protective immunity. Although salivary testing has been used for many viral infections, including severe acute respiratory syndrome (SARS) and Middle East Respiratory Syndrome (MERS), its use has not yet been well established with the SARS coronavirus 2 (SARS-CoV-2). Quantification of salivary IgA and IgG antibodies can elucidate mucosal and systemic immune responses after natural infection or vaccination. Here, we report the development and validation of a rapid enzyme-linked immunosorbent assay (ELISA) for anti-SARS-CoV-2 salivary IgA and serum IgG antibodies, and present quantitative results for immunized subjects both prior to or following COVID-19 infections. Objective: Total and serum SARS-CoV-2 spike-specific IgG responses were compared with salivary spike-specific IgA and IgG responses in samples obtained from patients recently infected with SARS-CoV-2 and from subjects recently immunized with COVID-19 vaccines. Methods: A total of 52 paired saliva and serum samples were collected from 26 study participants: 7 subjects after COVID-19 infection and 19 subjects who were uninfected. The ELISA results from these samples were compared with five prepandemic control serum samples. Total IgG and SARS-CoV-2 spike-specific IgG in the serum samples from the subjects who were infected and vaccinated were also measured in a commercial laboratory with an enzyme immunoassay. Results: A wide variation in antibody responses was seen in salivary and serum samples measured by both methods. Three groups of serum total and IgG spike-specific SARS-CoV-2 antibody responses were observed: (1) low, (2) intermediate, and (3) high antibody responders. A correlational analysis of salivary IgA (sIgA) responses with serum IgG concentrations showed a statistical correlation in the low and intermediate antibody responder groups but not in the high group (which we believe was a result of saturation). Conclusion: These preliminary findings suggest measuring salivary and serum IgG and IgA merit further investigation as markers of current or recent SARS-CoV-2 infections.
Conflict of interest statement
The authors have no conflicts of interest to declare pertaining to this article
Figures




Comment in
-
Salivary and serum IgA and IgG after COVID-19 and after immunization with COVID-19 vaccines.Allergy Asthma Proc. 2023 Jan 1;44(1):e1. doi: 10.2500/aap.2023.44.220086. Allergy Asthma Proc. 2023. PMID: 36719698 No abstract available.
References
-
- Tomasi TB, Jr, DeCoteau E. Mucosal antibodies in respiratory and gastrointestinal disease. Adv Intern Med. 1970; 116:401–425. - PubMed
-
- Bellanti JA, Artenstein MS, Buescher EL. Characterization of virus neutralizing antibodies in human serum and nasal secretions. J Immunol. 1965; 94:344–351. - PubMed
-
- Smith CB, Bellanti JA, Chanock RM. Immunoglobulins in serum and nasal secretions following infection with type 1 parainfluenza virus and injection of inactivated vaccines. J Immunol. 1967; 99:133–141. - PubMed
-
- Bellanti JA. Role of local gamma-A-immunoglobulins in immunity. Am J Dis Child. 1968; 115:239–246. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

