Real-world experience with weight gain among pregnant women living with HIV who are using integrase inhibitors
- PMID: 36065478
- PMCID: PMC9985658
- DOI: 10.1111/hiv.13388
Real-world experience with weight gain among pregnant women living with HIV who are using integrase inhibitors
Abstract
Objectives: We assessed real-world weight change and pregnancy outcomes among pregnant women living with HIV who used integrase strand transferase inhibitor (INSTI)-based combined antiretroviral therapy (cART).
Methods: In a retrospective cohort study from 2014 to 2021 for prevention of perinatal HIV infection, we evaluated changes in weight from the first prenatal visit to near delivery for two groups. The categories of change were: low (< 0.18 kg/week), normal (0.18-0.59 kg/week), and high (> 0.59 kg/week). The backbones were lamivudine + tenofovir disoproxil or lamivudine + zidovudine. The comparison groups were women with body mass index (BMI) < 25 kg/m2 versus BMI ≥ 25 kg/m2 and INSTI-naïve versus INSTI-experienced. Continuous variables were analysed with a Kruskal-Wallis test and count or categorical data with χ2 tests.
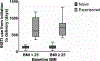
Results: We enrolled 198 pregnant women. At study entry, 74 had BMI < 25 kg/m2 and 124 had BMI ≥ 25 kg/m2 . Excess gestational weight gain was more frequent among women who were INSTI-naïve among both BMI groups (< 25 and ≥ 25). However, the proportion of participants per weight change category was only significantly different between INSTI-naïve women with baseline BMI < 25 kg/m2 and INSTI-experienced women with BMI < 25 kg/m2 . In particular, INSTI-naïve women with BMI < 25 kg/m2 had significantly higher rates of excess gestational weight gain (31.6%) compared with participants with BMI < 25 kg/m2 who conceived while on INSTIs (11.8%, p = 0.004). Rates of unfavourable pregnancy outcomes were low and did not differ significantly between groups.
Conclusions: INSTI-naïve participants with BMI < 25 kg/m2 gained more weight during pregnancy than participants with BMI ≥ 25 kg/m2 who conceived while using INSTIs. Rates of adverse pregnancy outcomes did not differ between the groups.
Keywords: HIV integrase inhibitors; anti-HIV agents; gestational weight gain; pregnancy outcomes.
© 2022 British HIV Association.
Conflict of interest statement
Figures

Similar articles
-
Associations between change in BMI and the risk of hypertension and dyslipidaemia in people receiving integrase strand-transfer inhibitors, tenofovir alafenamide, or both compared with other contemporary antiretroviral regimens: a multicentre, prospective observational study from the RESPOND consortium cohorts.Lancet HIV. 2024 May;11(5):e321-e332. doi: 10.1016/S2352-3018(23)00328-4. Epub 2024 Apr 12. Lancet HIV. 2024. PMID: 38621392 Free PMC article.
-
The Effect of Antiretroviral Therapy for the Treatment of Human Immunodeficiency Virus (HIV)-1 in Pregnancy on Gestational Weight Gain.Clin Infect Dis. 2022 Sep 10;75(4):665-672. doi: 10.1093/cid/ciab994. Clin Infect Dis. 2022. PMID: 34864949
-
An assessment of weight change associated with the initiation of a protease or integrase strand transfer inhibitor in patients with human immunodeficiency virus.Curr Med Res Opin. 2020 Aug;36(8):1313-1323. doi: 10.1080/03007995.2020.1775074. Epub 2020 Jun 17. Curr Med Res Opin. 2020. PMID: 32459155
-
A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection.Retrovirology. 2022 Oct 22;19(1):22. doi: 10.1186/s12977-022-00608-1. Retrovirology. 2022. PMID: 36273165 Free PMC article. Review.
-
Abacavir/dolutegravir/lamivudine single-tablet regimen: a review of its use in HIV-1 infection.Drugs. 2015 Apr;75(5):503-14. doi: 10.1007/s40265-015-0361-6. Drugs. 2015. PMID: 25698454 Review.
Cited by
-
Impact of Protease Inhibitor-Based Highly Active Antiretroviral Therapy on Fetal Subcutaneous Fat Tissue in HIV-Pregnant Women in a Middle-Income Country.Viruses. 2023 Dec 20;16(1):10. doi: 10.3390/v16010010. Viruses. 2023. PMID: 38275945 Free PMC article.
References
-
- World Health Organization. Updated Recommendations on HIV Prevention, Infant Diagnosis, Antiretroviral Initiation and Monitoring. Geneva: WHO; 2021. - PubMed
-
- U.S. Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents Panel Members and Consultants. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Washington, DC: U. S. Department of Health and Human Services; 2021.
-
- European AIDS Clinical Society. Guidelines. Version 11.0. Brussels, Belgium: EACS; 2021.
-
- British HIV Association. BHIVA guidelines on antiretroviral treatment for adults living with HIV-1 2022 - consultation version. London: BHIVA; 2022.
-
- Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. New England Journal of Medicine. 2019; 381:803–15. - PubMed

