Incidence and Risk Factors for Totally Implantable Venous Access Device Infections in Pediatric Patients With Cancer: A Study of 25,954 Device-Days
- PMID: 36065650
- PMCID: PMC9444570
- DOI: 10.3346/jkms.2022.37.e266
Incidence and Risk Factors for Totally Implantable Venous Access Device Infections in Pediatric Patients With Cancer: A Study of 25,954 Device-Days
Abstract
Background: Totally implantable venous access devices (TIVADs) are frequently used in pediatric patients with cancer owing to their multiple benefits. Despite occasional infections with TIVADs, knowledge of the incidence and risk factors is limited.
Methods: This retrospective study included pediatric patients with cancer who received TIVAD at Chungbuk National University Hospital from 2001 to 2021. We collected data on demographics, diagnosis, duration of TIVAD use, pathogens, and other risk factors.
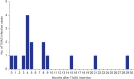
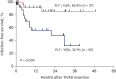
Results: During the study period, 55 TIVADs with 25,954 device-days were applied in 49 patients. There were 15 TIVAD infections (15/55, 27.3%), with an infection rate of 0.21 infections per TIVAD per year (0.58 cases/1,000 device-days). TIVAD infections occurred at a median of 5 months (range, 8 days-30 months) after insertion. The most common causative microorganisms were methicillin-resistant coagulase-negative staphylococci (n = 8, 53.3%) followed by Escherichia coli (n = 3, 20.0%). Infection-free TIVAD survival was higher in the group with normal platelet count at insertion (platelet counts ≥ 150,000/μL) than in the group with thrombocytopenia at insertion (platelet counts < 150,000/μL) (81.3% vs. 32.1%, P = 0.004). Device removal was the mainstay of treatment (11/15, 73.3%).
Conclusion: TIVAD infection may be related to thrombocytopenia at the time of device insertion. Further studies are needed to identify preventive factors against TIVAD infections in children with cancer.
Keywords: Acute Lymphoblastic Leukemia; Children; Infection; Pediatric Cancer; Totally Implantable Venous Access Device.
© 2022 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
Similar articles
-
The role of antibiotics in preventing totally implantable venous access device (TIVAD) infections; is there a population that would benefit?Clin Imaging. 2018 Sep-Oct;51:213-216. doi: 10.1016/j.clinimag.2018.05.012. Epub 2018 May 28. Clin Imaging. 2018. PMID: 29864730
-
Does systemic antibiotic prophylaxis prior to the placement of totally implantable venous access devices reduce early infection? A retrospective study of 1,485 cases at a large academic institution.Am J Infect Control. 2020 Jan;48(1):95-99. doi: 10.1016/j.ajic.2019.06.028. Epub 2019 Aug 20. Am J Infect Control. 2020. PMID: 31439370
-
Assessing the time-to-removal of totally implantable venous access devices comparing valved-versus open-ended catheters in patients treated with chemotherapy.J Vasc Access. 2025 Mar;26(2):447-454. doi: 10.1177/11297298231223539. Epub 2024 Jan 11. J Vasc Access. 2025. PMID: 38205615
-
Surgical placement of totally implantable venous access device-an institutional experience.Indian J Pediatr. 2014 Sep;81(9):866-70. doi: 10.1007/s12098-013-1183-8. Epub 2013 Aug 14. Indian J Pediatr. 2014. PMID: 23943575 Review.
-
Chinese expert consensus on the nursing management of the totally implantable venous access device.J Cancer Res Ther. 2022 Sep;18(5):1231-1240. doi: 10.4103/jcrt.jcrt_387_22. J Cancer Res Ther. 2022. PMID: 36204867 Review.
Cited by
-
Totally implantable venous ports in infants and children: a single-center retrospective study of indications and safety.Front Oncol. 2024 Apr 16;14:1351630. doi: 10.3389/fonc.2024.1351630. eCollection 2024. Front Oncol. 2024. PMID: 38690159 Free PMC article.
-
Inversion of Central Venous Ports in Children Under Six Years Old: A Retrospective Analysis of 154 Oncology Patients.Cureus. 2024 Jun 25;16(6):e63106. doi: 10.7759/cureus.63106. eCollection 2024 Jun. Cureus. 2024. PMID: 39055458 Free PMC article.
References
-
- Stovroff M, Teague WG. Intravenous access in infants and children. Pediatr Clin North Am. 1998;45(6):1373–1393. viii. - PubMed
-
- Jung SE, Kim YH, Jun YS, Kim DY, Park JK, Lee SC, et al. Totally implantable venous access devices in pediatric surgery patients. J Korean Surg Soc. 1997;52(3):420–425.
-
- Di Carlo I, Cordio S, La Greca G, Privitera G, Russello D, Puleo S, et al. Totally implantable venous access devices implanted surgically: a retrospective study on early and late complications. Arch Surg. 2001;136(9):1050–1053. - PubMed
-
- Fratino G, Molinari AC, Parodi S, Longo S, Saracco P, Castagnola E, et al. Central venous catheter-related complications in children with oncological/hematological diseases: an observational study of 418 devices. Ann Oncol. 2005;16(4):648–654. - PubMed
-
- Alexander N. Question 3. Do Portacaths or Hickman lines have a higher risk of catheter-related bloodstream infections in children with leukaemia? Arch Dis Child. 2010;95(3):239–241. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical