Nelonemdaz for Patients With Acute Ischemic Stroke Undergoing Endovascular Reperfusion Therapy: A Randomized Phase II Trial
- PMID: 36065810
- PMCID: PMC9586831
- DOI: 10.1161/STROKEAHA.122.039649
Nelonemdaz for Patients With Acute Ischemic Stroke Undergoing Endovascular Reperfusion Therapy: A Randomized Phase II Trial
Abstract
Background: Nelonemdaz is a multitarget neuroprotectant that selectively blocks N-methyl-D-aspartate receptors and scavenges free radicals, as proven in preclinical ischemia-reperfusion studies. We aimed to evaluate the safety and efficacy of nelonemdaz in patients with acute ischemic stroke receiving endovascular reperfusion therapy.
Methods: This phase II randomized trial involved participants with large-artery occlusion in the anterior circulation at baseline who received endovascular reperfusion therapy <8 hours from symptom onset at 7 referral stroke centers in South Korea between October 29, 2016, and June 1, 2020. Two hundred thirteen patients were screened and 209 patients were randomly assigned at a 1:1:1 ratio using a computer-generated randomization system. Patients were divided into 3 groups based on the medication received-placebo, low-dose (2750 mg) nelonemdaz, and high-dose (5250 mg) nelonemdaz. The primary outcome was the proportion of patients with modified Rankin Scale scores of 0-2 at 12 weeks.
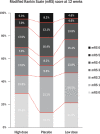
Results: Two hundred eight patients were assigned to the placebo (n=70), low-dose (n=71), and high-dose (n=67) groups. The groups had similar baseline characteristics. The primary outcome was achieved in 183 patients, and it did not differ among the groups (33/61 [54.1%], 40/65 [61.5%], and 36/57 [63.2%] patients; P=0.5578). The common odds ratio (90% CI) indicating a favorable shift in the modified Rankin Scale scores at 12 weeks was 1.55 (0.92-2.60) between the placebo and low-dose groups and 1.61 (0.94-2.76) between the placebo and high-dose groups. No serious adverse events were reported.
Conclusions: The study arms showed no significant difference in the proportion of patients achieving modified Rankin Scale scores of 0-2 at 12 weeks. Nevertheless, nelonemdaz-treated patients showed a favorable tendency toward achieving these scores at 12 weeks, without serious adverse effects. Thus, a large-scale phase III trial is warranted.
Registration: URL: https://clinicaltrials.gov; Unique identifier: NCT02831088.
Keywords: cerebral infarction; ischemic stroke; neuroprotective agent; odds ratio; reperfusion.
Figures

References
-
- Gwag BJ, Lee YA, Ko SY, Lee MJ, Im DS, Yun BS, Lim HR, Park SM, Byun HY, Son SJ, et al. Marked prevention of ischemic brain injury by Neu2000, an NMDA antagonist and antioxidant derived from aspirin and sulfasalazine. J Cereb Blood Flow Metab. 2007;27:1142–1151. doi: 10.1038/sj.jcbfm.9600418 - PubMed
-
- Gwag BJ, Koh JY, DeMaro JA, Ying HS, Jacquin M, Choi DW. Slowly triggered excitotoxicity occurs by necrosis in cortical cultures. Neuroscience. 1997;77:393–401. doi: 10.1016/s0306-4522(96)00473-3 - PubMed
-
- Visavadiya NP, McEwen ML, Pandya JD, Sullivan PG, Gwag BJ, Springer JE. Antioxidant properties of Neu2000 on mitochondrial free radicals and oxidative damage. Toxicol In Vitro. 2013;27:788–797. doi: 10.1016/j.tiv.2012.12.011 - PubMed
-
- Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, et al. ; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X - PubMed
-
- Badhiwala JH, Nassiri F, Alhazzani W, Selim MH, Farrokhyar F, Spears J, Kulkarni AV, Singh S, Alqahtani A, Rochwerg B, et al. Endovascular thrombectomy for acute ischemic stroke: a meta-analysis. JAMA. 2015;314:1832–1843. doi: 10.1001/jama.2015.13767 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

