Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children
- PMID: 36065889
- PMCID: PMC9446385
- DOI: 10.1002/14651858.CD013359.pub3
Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children
Update in
-
Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children.Cochrane Database Syst Rev. 2025 Oct 23;10(10):CD013359. doi: 10.1002/14651858.CD013359.pub4. Cochrane Database Syst Rev. 2025. PMID: 41128098 Free PMC article.
Abstract
Background: Every year, an estimated one million children and young adolescents become ill with tuberculosis, and around 226,000 of those children die. Xpert MTB/RIF Ultra (Xpert Ultra) is a molecular World Health Organization (WHO)-recommended rapid diagnostic test that simultaneously detects Mycobacterium tuberculosis complex and rifampicin resistance. We previously published a Cochrane Review 'Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for tuberculosis disease and rifampicin resistance in children'. The current review updates evidence on the diagnostic accuracy of Xpert Ultra in children presumed to have tuberculosis disease. Parts of this review update informed the 2022 WHO updated guidance on management of tuberculosis in children and adolescents.
Objectives: To assess the diagnostic accuracy of Xpert Ultra for detecting: pulmonary tuberculosis, tuberculous meningitis, lymph node tuberculosis, and rifampicin resistance, in children with presumed tuberculosis. Secondary objectives To investigate potential sources of heterogeneity in accuracy estimates. For detection of tuberculosis, we considered age, comorbidity (HIV, severe pneumonia, and severe malnutrition), and specimen type as potential sources. To summarize the frequency of Xpert Ultra trace results.
Search methods: We searched the Cochrane Infectious Diseases Group Specialized Register, MEDLINE, Embase, three other databases, and three trial registers without language restrictions to 9 March 2021.
Selection criteria: Cross-sectional and cohort studies and randomized trials that evaluated Xpert Ultra in HIV-positive and HIV-negative children under 15 years of age. We included ongoing studies that helped us address the review objectives. We included studies evaluating sputum, gastric, stool, or nasopharyngeal specimens (pulmonary tuberculosis), cerebrospinal fluid (tuberculous meningitis), and fine needle aspirate or surgical biopsy tissue (lymph node tuberculosis). For detecting tuberculosis, reference standards were microbiological (culture) or composite reference standard; for stool, we also included Xpert Ultra performed on a routine respiratory specimen. For detecting rifampicin resistance, reference standards were drug susceptibility testing or MTBDRplus.
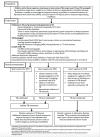
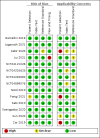
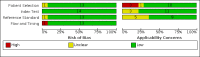
Data collection and analysis: Two review authors independently extracted data and, using QUADAS-2, assessed methodological quality judging risk of bias separately for each target condition and reference standard. For each target condition, we used the bivariate model to estimate summary sensitivity and specificity with 95% confidence intervals (CIs). We stratified all analyses by type of reference standard. We summarized the frequency of Xpert Ultra trace results; trace represents detection of a very low quantity of Mycobacterium tuberculosis DNA. We assessed certainty of evidence using GRADE.
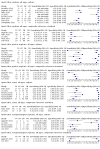
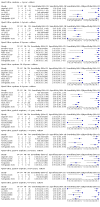
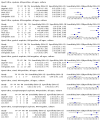
Main results: We identified 14 studies (11 new studies since the previous review). For detection of pulmonary tuberculosis, 335 data sets (25,937 participants) were available for analysis. We did not identify any studies that evaluated Xpert Ultra accuracy for tuberculous meningitis or lymph node tuberculosis. Three studies evaluated Xpert Ultra for detection of rifampicin resistance. Ten studies (71%) took place in countries with a high tuberculosis burden based on WHO classification. Overall, risk of bias was low. Detection of pulmonary tuberculosis Sputum, 5 studies Xpert Ultra summary sensitivity verified by culture was 75.3% (95% CI 64.3 to 83.8; 127 participants; high-certainty evidence), and specificity was 97.1% (95% CI 94.7 to 98.5; 1054 participants; high-certainty evidence). Gastric aspirate, 7 studies Xpert Ultra summary sensitivity verified by culture was 70.4% (95% CI 53.9 to 82.9; 120 participants; moderate-certainty evidence), and specificity was 94.1% (95% CI 84.8 to 97.8; 870 participants; moderate-certainty evidence). Stool, 6 studies Xpert Ultra summary sensitivity verified by culture was 56.1% (95% CI 39.1 to 71.7; 200 participants; moderate-certainty evidence), and specificity was 98.0% (95% CI 93.3 to 99.4; 1232 participants; high certainty-evidence). Nasopharyngeal aspirate, 4 studies Xpert Ultra summary sensitivity verified by culture was 43.7% (95% CI 26.7 to 62.2; 46 participants; very low-certainty evidence), and specificity was 97.5% (95% CI 93.6 to 99.0; 489 participants; high-certainty evidence). Xpert Ultra sensitivity was lower against a composite than a culture reference standard for all specimen types other than nasopharyngeal aspirate, while specificity was similar against both reference standards. Interpretation of results In theory, for a population of 1000 children: • where 100 have pulmonary tuberculosis in sputum (by culture): - 101 would be Xpert Ultra-positive, and of these, 26 (26%) would not have pulmonary tuberculosis (false positive); and - 899 would be Xpert Ultra-negative, and of these, 25 (3%) would have tuberculosis (false negative). • where 100 have pulmonary tuberculosis in gastric aspirate (by culture): - 123 would be Xpert Ultra-positive, and of these, 53 (43%) would not have pulmonary tuberculosis (false positive); and - 877 would be Xpert Ultra-negative, and of these, 30 (3%) would have tuberculosis (false negative). • where 100 have pulmonary tuberculosis in stool (by culture): - 74 would be Xpert Ultra-positive, and of these, 18 (24%) would not have pulmonary tuberculosis (false positive); and - 926 would be Xpert Ultra-negative, and of these, 44 (5%) would have tuberculosis (false negative). • where 100 have pulmonary tuberculosis in nasopharyngeal aspirate (by culture): - 66 would be Xpert Ultra-positive, and of these, 22 (33%) would not have pulmonary tuberculosis (false positive); and - 934 would be Xpert Ultra-negative, and of these, 56 (6%) would have tuberculosis (false negative). Detection of rifampicin resistance Xpert Ultra sensitivity was 100% (3 studies, 3 participants; very low-certainty evidence), and specificity range was 97% to 100% (3 studies, 128 participants; low-certainty evidence). Trace results Xpert Ultra trace results, regarded as positive in children by WHO standards, were common. Xpert Ultra specificity remained high in children, despite the frequency of trace results.
Authors' conclusions: We found Xpert Ultra sensitivity to vary by specimen type, with sputum having the highest sensitivity, followed by gastric aspirate and stool. Nasopharyngeal aspirate had the lowest sensitivity. Xpert Ultra specificity was high against both microbiological and composite reference standards. However, the evidence base is still limited, and findings may be imprecise and vary by study setting. Although we found Xpert Ultra accurate for detection of rifampicin resistance, results were based on a very small number of studies that included only three children with rifampicin resistance. Therefore, findings should be interpreted with caution. Our findings provide support for the use of Xpert Ultra as an initial rapid molecular diagnostic in children being evaluated for tuberculosis.
Copyright © 2022 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
AK has conducted prior primary research on tuberculosis diagnostics. The Baylor College of Medicine Children's Foundation‐Swaziland, where Dr Kay is based, received a discount from Cepheid on Xpert MTB/RIF Ultra cartridges for a tuberculosis case finding programme. The Baylor College of Medicine Children's Foundation‐Eswatini is separate from Baylor College of Medicine (AK's employer).
TN has no known conflicts of interest.
SEV is a Medical Officer at the World Health Organization Global Tuberculosis Programme, which commissioned this review update for the 2022 WHO consolidated guidelines on the management of tuberculosis in children and adolescents.
KV is a WHO staff member.
AB works as a technical officer at the WHO Global Tuberculoiss Programme, which commissioned this review update for the 2022 WHO consolidated guidelines on the management of tuberculosis in children and adolescents.
TM is a consultant to the WHO.
LGF has no known conflicts of interest.
ME is a CIDG Editor, and has no known conflicts of interest.
AKD has conducted prior primary research on tuberculosis diagnostics and has no known conflicts of interest. She works for UNICEF, and in her role as child health specialist is sometimes involved in developing recommendations for diagnostic approaches to detect childhood illnesses.
AMM has conducted prior primary research on tuberculosis diagnostics and has no known conflicts of interest. She has undertaken work as an independent contractor for Janssen Global Services, Medscape Independent Contractor, and Oxford Immunotec Inc.
KRS has received financial support for the preparation of systematic reviews and educational materials, consultancy fees from the Foundation for Innovative New Diagnostics (FIND) (for the preparation of systematic reviews), honoraria, and travel support to attend WHO guidelines meetings. KRS is a CIDG and DTA Editor.
YT is a Cochrane Editorial Board Member; a CIDG and DTA Editor; and a Statistical Editor for the Cochrane Bone Joint and Muscle Trauma Group.
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Figures



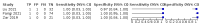

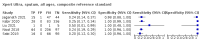

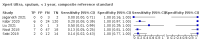


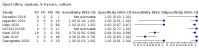
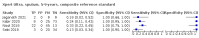

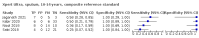

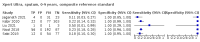
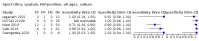
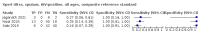


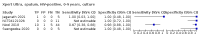
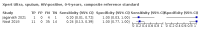


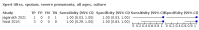

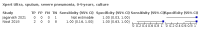
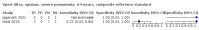



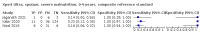
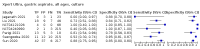
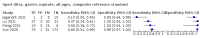

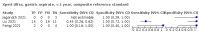
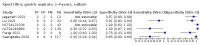
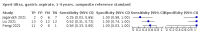



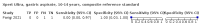
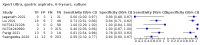

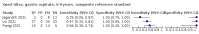





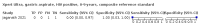
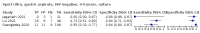

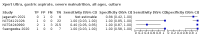

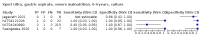
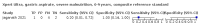
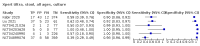

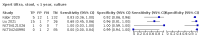
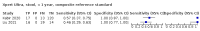
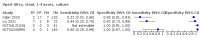
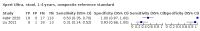




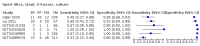
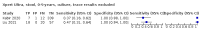
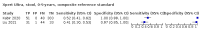
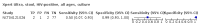
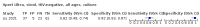
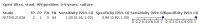
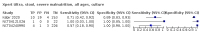
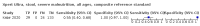
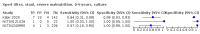
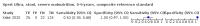
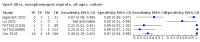
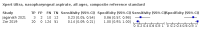

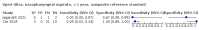
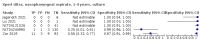
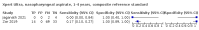


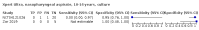

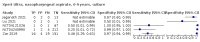

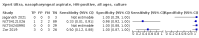
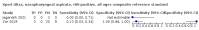
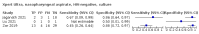


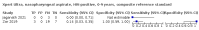
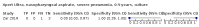

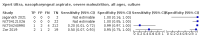
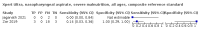
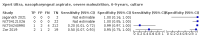
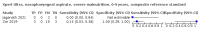
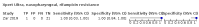
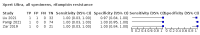
Update of
-
Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD013359. doi: 10.1002/14651858.CD013359.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2022 Sep 6;9:CD013359. doi: 10.1002/14651858.CD013359.pub3. PMID: 32853411 Free PMC article. Updated.
References
References to studies included in this review
Barcellini 2019 {published data only}
-
- Barcellini L, Borroni E, Cimaglia C, Girardi E, Matteelli A, Marchese V, et al. App-based symptoms screening with Xpert MTB/RIF Ultra assay used for active tuberculosis detection in migrants at point of arrivals in Italy: the E-DETECT TB intervention analysis. PLOS One 2019;14(7):e0218039. - PMC - PubMed
Jaganath 2021 {published data only}
Kabir 2020 {published data only}
Liu 2021 {published data only}
-
- Liu XH, Xia L, Song B, Wang H, Qian XQ, Wei JH, et al. Stool-based Xpert MTB/RIF Ultra assay as a tool for detecting pulmonary tuberculosis in children with abnormal chest imaging: a prospective cohort study. Journal of Infection 2021;82(1):84-9. - PubMed
NCT04121026 {unpublished data only}
-
- NCT04121026. Validation of a tuberculosis treatment decision algorithm in HIV-infected children (TB-Speed HIV). clinicaltrials.gov/show/NCT04121026 (first received 9 October 2019).
NCT04203628 {unpublished data only}
-
- NCT04203628. Evaluation of four stool processing methods combined with Xpert MTB/RIF Ultra for diagnosis of intrathoracic paediatric tuberculosis. clinicaltrials.gov/ct2/show/NCT04203628 (first received 18 December 2019).
NCT04240990 {unpublished data only}
-
- NCT04240990. Development of a diagnostic prediction score for tuberculosis in hospitalized children with severe acute malnutrition (TB-Speed SAM). clinicaltrials.gov/show/NCT04240990 (first received 27 January 2020).
NCT04899076 {published data only}
-
- NCT04899076. Stool Processing Kit (SPK) evaluation for paediatric TB. clinicaltrials.gov/ct2/show/NCT04899076 (first received 24 May 2021).
Nicol 2018 {published data only}
-
- Nicol MP, Workman L, Prins M, Bateman L, Ghebrekristos Y, Mbhele S, et al. Accuracy of Xpert MTB/RIF Ultra for the diagnosis of pulmonary tuberculosis in children. Paediatric Infectious Disease Journal 2018;37(10):e261-3. - PubMed
Parigi 2021 {published data only}
-
- Parigi S, Venturini E, Galli L, Chiappini E. Xpert MTB/RIF Ultra performance in diagnosing paediatric pulmonary TB in gastric aspirates. International Journal of Tuberculosis and Lung Disease 2021;25(1):75-7. - PubMed
Sabi 2018 {published data only}
-
- Sabi I, Rachow A, Mapamba D, Clowes P, Ntinginya NE, Sasamalo M, et al. Xpert MTB/RIF Ultra assay for the diagnosis of pulmonary tuberculosis in children: a multicentre comparative accuracy study. Journal of Infection 2018;77(4):321-7. - PubMed
Ssengooba 2020 {published data only}
Sun 2020 {published data only}
Zar 2019 {published data only}
References to studies excluded from this review
Ali 2017 {published data only}
-
- Ali RH, Ibrahim NY, Elegail AM, Eltohami NA, Ebraheem RS, Ahmed SF, et al. Evaluation of GeneXpert MTB/RIF and line probe assay for rapid diagnosis of Mycobacterium tuberculosis in Sudanese pulmonary TB patients. Asian Pacific Journal of Tropical Disease 2017;7(7):426-9.
Atashi 2017 {published data only}
Atehortúa Muñoz 2017 {published data only}
-
- Atehortúa Muñoz SL, Muñoz JR, Cárdenas Moreno SV, Ferreira CA, Cornejo Ochoa JW. Xpert MTB/RIF as a diagnostic tool in a cohort of children under 15 years of age with clinical suspicion of pulmonary tuberculosis in a hospital of high complexity in Medellin [Xpert MTB/RIF® como herramienta diagnóstica en una cohorte de niños menores de 15 años con sospecha clínica de tuberculosis pulmonar en un hospital de alta complejidad de Medellín]. Infectio 2017;21(1):25-31.
Azevedo 2018 {published data only}
Ballif 2015 {published data only}
Banada 2016 {published data only}
Biadglegne 2014 {published data only}
-
- Biadglegne F, Mulu A, Rodloff AC, Sack U. Diagnostic performance of the Xpert MTB/RIF assay for tuberculous lymphadenitis on fine needle aspirates from Ethiopia. Tuberculosis (Edinburgh, Scotland) 2014;94(5):502-5. - PubMed
Bojang 2016 {published data only}
-
- Bojang AL, Mendy FS, Tientcheu LD, Otu J, Antonio M, Kampmann B, et al. Comparison of TB-LAMP, GeneXpert MTB/RIF and culture for diagnosis of pulmonary tuberculosis in The Gambia. Journal of Infection 2016;72(3):332-7. - PubMed
Che 2017 {published data only}
-
- Che NY, Huang SJ, Ma Y, Han Y, Liu ZC, Zhang C, et al. Comparison of histological, microbiological, and molecular methods in diagnosis of patients with TBLN having different anti-TB treatment background. Biomedical and Environmental Sciences 2017;30(6):418-25. - PubMed
Cox 2014 {published data only}
Cross 2014 {published data only}
Diallo 2016 {published data only}
-
- Diallo AB, Kollo AI, Camara M, Lo S, Ossoga GW, Mbow M, et al. Performance of GeneXpert MTB/RIF in the diagnosis of extrapulmonary tuberculosis in Dakar: 2010–2015 [Performance du GeneXpert MTB/RIF ® dans le diagnostic de la tuberculose extra-pulmonaire à Dakar: 2010–2015]. Pan African Medical Journal 2016;25:129. - PMC - PubMed
DiNardo 2016 {published data only}
DiNardo 2018 {published data only}
Ejeh 2018 {published data only}
-
- Ejeh EF, Undiandeye A, Akinseye VO, Okon KO, Kazeem HM, Kudi CA, et al. Diagnostic performance of GeneXpert and Ziehl-Neelson microscopy in the detection of tuberculosis in Benue State, Nigeria. Alexandria Journal of Medicine 2018;54(4):529-33.
Gautam 2018 {published data only}
-
- Gautam H, Agrawal SK, Verma SK, Singh UB. Cervical tuberculous lymphadenitis: clinical profile and diagnostic modalities. International Journal of Mycobacteriology 2018;7(3):212-6. - PubMed
Gelalcha 2017 {published data only}
Geleta 2015 {published data only}
Ghariani 2015 {published data only}
-
- Ghariani A, Jaouadi T, Smaoui S, Mehiri E, Marouane C, Kammoun S, et al. Diagnosis of lymph node tuberculosis using the GeneXpert MTB/RIF in Tunisia. International Journal of Mycobacteriology 2015;4(4):270-5. - PubMed
Giang 2015 {published data only}
Guajardo‐Lara 2018 {published data only}
-
- Guajardo-Lara CE, Saldaña-Ramírez MI, Hernández-Galván NN, Dimas-Adame MA, Ayala-Gaytán JJ, Valdovinos-Chávez SB. MGIT and other methods for diagnosing tuberculosis in a private hospital system with low incidence [MGIT y otros métodos para diagnosticar tuberculosis en un sistema hospitalario privado con baja incidencia]. Revista Médica del Instituto Mexicano del Seguro Social 2018;56(2):158-62. - PubMed
Gulla 2019 {published data only}
-
- Gulla KM, Gunathilaka G, Jat KR, Sankar J, Karan M, Lodha R, et al. Utility and safety of endobronchial ultrasound-guided transbronchial needle aspiration and endoscopic ultrasound with an echobronchoscope-guided fine needle aspiration in children with mediastinal pathology. Pediatric Pulmonology 2019;54(6):881-5. - PubMed
Hakim 2017 {published data only}
Helb 2010 {published data only}
Horo 2017 {published data only}
-
- Horo K, N'Guessan R, Koffi MO, Kouamé-N'Takpé N, Koné A, Samaké K, et al. Use of the Xpert® MTB/RIF test in routine screening of new cases of pulmonary tuberculosis in an endemic area. Revue des Maladies Respiratoires 2017;4(7):749-57. - PubMed
Huh 2014 {published data only}
Kuyinu 2018 {published data only}
-
- Kuyinu YA, Odugbemi BA, Salisu-Olatunji SO, Adepoju FO, Odusanya OO. Characteristics of Mycobacterium tuberculosis positive patients screened for drug-resistant tuberculosis at a tertiary health facility in Lagos, Nigeria. Journal of the National Medical Association 2018;110(1):88-91. - PubMed
Lopez 2019 {published data only}
-
- Lopez AL, Aldaba JG, Morales-Dizon M, Sarol JN, Daag JV, Ama MC, et al. Urine Xpert MTB/RIF for the diagnosis of childhood tuberculosis. International Journal of Infectious Diseases 2019;79:44-6. - PubMed
Lu J 2017 {published data only}
Lu Y 2018 {published data only}
-
- Lu Y, Zhu Y, Shen N, Tian L, Sun Z. Evaluating the diagnostic accuracy of the Xpert MTB/RIF assay on bronchoalveolar lavage fluid: a retrospective study. International Journal of Infectious Diseases 2018;71:14-9. - PubMed
Malik 2018 {published data only}
-
- Malik AA, Amanullah F, Codlin AJ, Siddiqui S, Jaswal M, Ahmed JF, et al. Improving childhood tuberculosis detection and treatment through facility-based screening in rural Pakistan. International Journal of Tuberculosis and Lung Disease 2018;22(8):851-7. - PubMed
Marcy 2018 {published data only}
-
- Marcy O, Tejiokem M, Msellati P, Truong Huu K, Do Chau V, Tran Ngoc D, et al. Mortality and its determinants in antiretroviral treatment-naive HIV-infected children with suspected tuberculosis: an observational cohort study. Lancet HIV 2018;5(2):e87-95. - PubMed
Masenga 2017 {published data only}
Mekonnen 2015 {published data only}
Memon 2018 {published data only}
-
- Memon SS, Sinha S, Sharma SK, Kabra SK, Lodha R, Soneja M. Diagnostic accuracy of Xpert Mtb/Rif assay in stool samples in intrathoracic childhood tuberculosis. Journal Tuberculosis and Therapeutics 2018;3(2):115.
Metaferia 2018 {published data only}
Mijovic 2018 {published data only}
-
- Mijovic H, Al-Nasser Y, Al-Rawahi G, Roberts A. Experience with using rapid molecular testing in diagnosing pulmonary and extra-pulmonary pediatric tuberculosis in a non-endemic setting – a retrospective case series. Paediatrics and Child Health (Canada) 2018;23(Suppl 1):e44-5.
Modi 2016 {published data only}
Mulenga 2015 {published data only}
Naidoo 2016 {published data only}
Nair 2016 {published data only}
Nansumba 2016 {published data only}
-
- Nansumba M, Kumbakumba E, Orikiriza P, Muller Y, Nackers F, Debeaudrap P, et al. Detection yield and tolerability of string test for diagnosis of childhood intrathoracic tuberculosis. Paediatric Infectious Disease Journal 2016;35(2):146-51. - PubMed
Nataprawira 2016 {published data only}
-
- Nataprawira HM, Ruslianti V, Solek R, Hawani D, Mianti M, Anggraeni R, et al. Outcome of tuberculous meningitis in children: the first comprehensive retrospective cohort study in Indonesia. International Journal of Tuberculosis and Lung Disease 2016;20(7):909-14. - PubMed
NCT03831906 {unpublished data only}
-
- NCT03831906. TB-Speed Pneumonia. clinicaltrials.gov/show/NCT03831906 (first received 6 February 2019).
NCT04038632 {unpublished data only}
-
- NCT04038632. TB-Speed Decentralisation Study. clinicaltrials.gov/show/NCT04038632 (first received 2 August 2019).
Ncube 2017 {published data only}
Nduba 2015 {published data only}
-
- Nduba V, Hoog AH, Mitchell E, Onyango P, Laserson K, Borgdorff M. Prevalence of tuberculosis in adolescents, western Kenya: implications for control programs. International Journal of Infectious Diseases 2015;35:11-7. - PubMed
Ngabonziza 2016 {published data only}
Nicol 2020 {published data only}
Ntinginya 2012 {published data only}
-
- Ntinginya EN, Squire SB, Millington KA, Mtafya B, Saathoff E, Heinrich N, et al. Performance of the Xpert® MTB/RIF assay in an active case-finding strategy: a pilot study from Tanzania. International Journal of Tuberculosis and Lung Disease 2012;16(11):1468-70. - PubMed
Opota 2019 {published data only}
Pandey 2017 {published data only}
Pink 2016 {published data only}
Planting 2014 {published data only}
-
- Planting NS, Visser GL, Nicol MP, Workman L, Isaacs W, Zar HJ. Safety and efficacy of induced sputum in young children hospitalised with suspected pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease 2014;18(1):8-12. - PubMed
Raizada 2014 {published data only}
-
- Raizada N, Sachdeva KS, Nair SA, Kulsange S, Gupta RS, Thakur R, et al. Enhancing TB case detection: experience in offering upfront Xpert MTB/RIF testing to pediatric presumptive TB and DR TB cases for early rapid diagnosis of drug sensitive and drug resistant TB. PLOS One 2014;9(8):e105346. - PMC - PubMed
Raizada 2015a {published data only}
Raizada 2015b {published data only}
Raizada 2018a {published data only}
Raizada 2018b {published data only}
Rathour 2019 {published data only}
-
- Rathour JS, Mantan M, Khanna A, et al. Evaluation of GENE XPERT assay in extrapulmonary tuberculosis in children. Journal of Evolution of Medical and Dental Sciences 2019;8(1):76-80.
Rebecca 2018 {published data only}
-
- Rebecca B, Chacko A, Verghese V, Rose W. Spectrum of pediatric tuberculosis in a tertiary care setting in South India. Journal of Tropical Pediatrics 2018;64(6):544-7. - PubMed
Rivera 2017 {published data only}
Sabi 2016 {published data only}
-
- Sabi I, Kabyemera R, Mshana SE, Kidenya BR, Kasanga G, Gerwing-Adima LE, et al. Pulmonary TB bacteriologically confirmed by induced sputum among children at Bugando Medical Centre, Tanzania. International Journal of Tuberculosis and Lung Disease 2016;20(2):228-34. - PubMed
Sachdeva 2015 {published data only}
Sanchini 2014 {published data only}
-
- Sanchini A, Fiebig L, Drobniewski F, Haas W, Richter E, Katalinic-Jankovic V, et al. Laboratory diagnosis of paediatric tuberculosis in the European Union/European Economic Area: analysis of routine laboratory data, 2007 to 2011. Eurosurveillance 2014;19(11):pii: 20744. - PubMed
Sander 2019 {published data only}
-
- Sander MS, Laah SN, Titahong CN, Lele C, Kinge T, Jong BC, et al. Systematic screening for tuberculosis among hospital outpatients in Cameroon: the role of screening and testing algorithms to improve case detection. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases 2019;15:100095. [DOI: 10.1016/j.jctube.2019.100095] - DOI - PMC - PubMed
Sanjuan‐Jimenez 2015 {published data only}
Schumacher 2016 {published data only}
Scott 2014 {published data only}
Shah 2016b {published data only}
-
- Shah I, Gupta Y. Xpert MTB/RIF for diagnosis of tuberculosis and drug resistance in Indian children. Indian Pediatrics 2016;53(9):837-8. - PubMed
Shah 2018 {published data only}
-
- Shah MA, Shah I. Increasing prevalence of pediatric drug-resistant tuberculosis in Mumbai, India, and its outcome. Pediatric Infectious Disease Journal 2018;37(12):1261-3. - PubMed
Shah 2019 {published data only}
-
- Shah I, Bhamre R, Shetty NS. Accuracy of Xpert® Mycobacterium tuberculosis/rifampicin assay in diagnosis of pulmonary tuberculosis. Infectious Diseases 2019;51(7):550-3. - PubMed
Sharma 2015 {published data only}
Sieiro 2018 {published data only}
-
- Sieiro TL, Aurílio RB, Soares EC, Chiang SS, Sant Anna CC. The role of the Xpert MTB/RIF assay among adolescents suspected of pulmonary tuberculosis in Rio de Janeiro, Brazil. Revista da Sociedade Brasileira de Medicina Tropical 2018;51(2):234-6. - PubMed
Singh 2015 {published data only}
Singh 2016 {published data only}
Solomons 2016 {published data only}
-
- Solomons RS, Visser DH, Marais BJ, Schoeman JF, Furth AM. Diagnostic accuracy of a uniform research case definition for TBM in children: a prospective study. International Journal of Tuberculosis and Lung Disease 2016;20(7):903-8. - PubMed
Sun 2019 {published data only}
-
- Sun L, Qi X, Liu F, Wu X, Yin Q, Guo Y, et al. A test for more accurate diagnosis of pulmonary tuberculosis. Pediatrics November 2019;144(5):e20190262. - PubMed
Sureshbabu 2016 {published data only}
-
- Sureshbabu R, Lakshmi Murali A, Palaniswamy M. Molecular diagnosis of drug resistance tuberculosis in the Districts Of Tamilnadu. International Journal of Pharma and Bio Sciences 2016;7(4):B42-B6.
Tadesse 2015 {published data only}
Tafur 2018 {published data only}
Tang 2017 {published data only}
Theron 2011 {published data only}
-
- Theron G, Peter J, Zyl-Smit R, Mishra H, Streicher E, Murray S, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. American Journal of Respiratory and Critical Care Medicine 2011;184(1):132-40. - PubMed
Triasih 2015 {published data only}
-
- Triasih R, Robertson C, Duke T, Graham SM. Risk of infection and disease with Mycobacterium tuberculosis among children identified through prospective community-based contact screening in Indonesia. Tropical Medicine & International Health 2015;20(6):737-43. - PubMed
Ullah 2017 {published data only}
-
- Ullah I, Javaid A, Masud H, Ali M, Basit A, Ahmad W, et al. Rapid detection of Mycobacterium tuberculosis and rifampicin resistance in extrapulmonary tuberculosis and sputum smear negative pulmonary suspects using Xpert MTB/RIF. Journal of Medical Microbiology 2017;66(4):412-8. - PubMed
Walters 2012 {published data only}
-
- Walters E, Gie RP, Hesseling AC, Friedrich SO, Diacon AH, Gie RP. Rapid diagnosis of pediatric intrathoracic tuberculosis from stool samples using the Xpert MTB/RIF assay: a pilot study. Pediatric Infectious Disease Journal 2012;31(12):1316. - PubMed
Walters 2017 {published data only}
Walters 2018 {published data only}
Wang 2020 {published data only}
-
- Wang G, Wang S, Yang X, Sun Q, Jiang G, Huang M, et al. Accuracy of Xpert MTB/RIF Ultra for the diagnosis of pleural TB in a multicenter cohort study. Chest 2020;157(2):268-275. - PubMed
Yadav 2020 {published data only}
-
- Yadav R, Vaidya P, Mathew JL, Singh S, Khaneja R, Agarwal P, et al. Diagnostic accuracy of Xpert MTB/RIF ultra for detection of Mycobacterium tuberculosis in children: a prospective cohort study. Letters in Applied Microbiology 2021;72(3):225-30. - PubMed
Zhang 2016 {published data only}
-
- Zhang AM, Li F, Liu XH, Xia L, Lu SH. Application of Gene Xpert Mycobacterium tuberculosis DNA and resistance to rifampicin assay in the rapid detection of tuberculosis in children. Zhonghua er ke za zhi [Chinese Journal of Pediatrics] 2016;54(5):370-4. - PubMed
References to ongoing studies
ChiCTR1800015075 {unpublished data only}
-
- ChiCTR1800015075. Diagnostic accuracy of Xpert MTB/RIF Ultra assay on diagnosing pediatric pulmonary tuberculosis. www.chictr.org.cn/showprojen.aspx?proj=25233 (first received 6 March 2018). [ChiCTR1800015075]
Additional references
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. - PubMed
Bjerrum 2019
Branigan 2021
-
- Branigan, D. Tuberculosis Diagnostics Pipeline report 2021. www.treatmentactiongroup.org/wp-content/uploads/2021/11/pipeline_TB_diag... (accessed 17 December 2021).
Broger 2019
Cepheid 2018
-
- Cepheid. Brochure: Xpert® MTB/RIF Ultra. www.cepheid.com/en/tests/Critical-Infectious-Diseases/Xpert-MTB-RIF-Ultra (accessed 3 November 2021).
Chakravorty 2017
Chiang 2017
-
- Chiang SS, Swanson DS, Starke JR. New diagnostics for childhood tuberculosis. Infectious Disease Clinics of North America 2015;29(3):477-502. - PubMed
Chu 2006
-
- Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331-2. - PubMed
Covidence [Computer program]
-
- Covidence. Melbourne, Australia: Veritas Health Innovation, (accessed 1 October 2021). Available at covidence.org.
Cruz 2012
-
- Cruz AT, Revell PA, Starke JR. Gastric aspirate yield for children with suspected pulmonary tuberculosis. Journal of the Pediatric Infectious Diseases Society 2012;2(2):171-4. - PubMed
David 2017
Dodd 2017
Dorman 2018
Dunn 2016
Frigati 2015
-
- Frigati L, Maskew M, Workman L, Munro J, Andronikou S, Nicol MP, et al. Predictors of culture-confirmed pulmonary tuberculosis in children in a high tuberculosis and HIV prevalence area. Pediatric Infectious Disease Journal 2015;34(9):e206-10. - PubMed
Furin 2019
-
- Furin J. Advances in the diagnosis, treatment, and prevention of tuberculosis in children. Expert Review of Respiratory Medicine 2019;13(3):301-11. - PubMed
Garner 2016
Graham 2012
-
- Graham SM, Ahmed T, Amanullah F, Browning R, Cardenas V, Casenghi M, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. Journal of Infectious Diseases 2012;205(Suppl 2):S199-208. - PMC - PubMed
Graham 2015
Harausz 2018
Horne 2019
Jenkins 2017
Kohli 2021
-
- Kohli M, Schiller I, Dendukuri N, Yao M, Dheda K, Denkinger CM, et al. Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD012768. [DOI: 10.1002/14651858.CD012768.pub3] - DOI - PMC - PubMed
Kunkel 2016
Macaskill 2010
-
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10. Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. Cochrane, 2013. Available from srdta.cochrane.org.
MacLean 2019
Marais 2004
-
- Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. International Journal of Tuberculosis and Lung Disease 2004;8(4):392-402. - PubMed
Marais 2005
Marais 2006a
-
- Marais BJ, Gie RP, Schaaf HS, Hesseling AS, Enarson DA, Beyers N. The spectrum of disease in children treated for tuberculosis in a highly endemic area. International Journal of Tuberculosis and Lung Disease 2006;10:732–8. - PubMed
Marais 2006b
-
- Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Enarson DA, Beyers N. The bacteriologic yield in children with intrathoracic tuberculosis. Clinical Infectious Diseases 2006;42:69-71. - PubMed
Marais 2006c
-
- Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Enarson DA, Beyers N. Radiographic signs and symptoms in children treated for tuberculosis: possible implications for symptom-based screening in resource-limited settings. Pediatric Infectious Disease Journal 2006;25(3):237-40. - PubMed
Marais 2006d
-
- Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Lombard C, Enarson DA, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics 2006;118(5):e1350-9. - PubMed
Marais 2006e
-
- Marais BJ, Wright CA, Schaaf HS, Gie RP, Hesseling AC, Enarson DA, et al. Tuberculous lymphadenitis as a cause of persistent cervical lymphadenopathy in children from a tuberculosis-endemic area. Pediatric Infectious Disease Journal 2006;25(2):142-6. - PubMed
Marais 2014
Marais S 2010
-
- Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infectious Diseases 2010;10(11):803-12. - PubMed
Mishra 2020
-
- Mishra H, Reeve BW, Palmer Z, Caldwell J, Dolby T, Naidoo CC, et al. Xpert MTB/RIF Ultra and Xpert MTB/RIF for diagnosis of tuberculosis in an HIV-endemic setting with a high burden of previous tuberculosis: a two-cohort diagnostic accuracy study. Lancet Respiratory Medicine 2020;8(4):368-82. - PubMed
Nathavitharana 2021
-
- Nathavitharana RR, Lederer P, Chaplin M, Bjerrum S, Steingart KR, Shah M. Impact of diagnostic strategies for tuberculosis using lateral flow urine lipoarabinomannan assay in people living with HIV. Cochrane Database of Systematic Reviews 2021, Issue 8. Art. No: CD014641. [DOI: 10.1002/14651858.CD014641] - DOI - PMC - PubMed
Newcombe 1998
-
- Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Statistics in Medicine 1998;17(8):873-90. - PubMed
Nicol 2014
Page 2021
Reid 2012
Reitsma 2015
-
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58:982-90. - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Schünemann 2008
Schünemann 2016
-
- Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, et al. GRADE Working Group. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. Journal of Clinical Epidemiology 2016;76:89-98. [DOI: 10.1016/j.jclinepi.2016.01.032] - DOI - PubMed
Schünemann 2020a
-
- Schünemann HJ, Mustafa R, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 1. Study design, risk of bias and indirectness in rating the certainty across a body of evidence for test accuracy. Journal of Clinical Epidemiology 2020;122:129-41. - PubMed
Schünemann 2020b
-
- Schünemann HJ, Mustafa R, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 2. Inconsistency, Imprecision, publication bias and other domains for rating the certainty of evidence for test accuracy and presenting it in evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2020;122:142-52. - PubMed
Shah 2016a
-
- Shah M, Hanrahan C, Wang ZY, Dendukuri N, Lawn SD, Denkinger CM, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD011420. [DOI: 10.1002/14651858.CD011420.pub2] - DOI - PMC - PubMed
Signorino 2022
Stata 16 [Computer program]
-
- Stata Statistical Software. StataCorp, Version 16. College Station: StataCorp LP, 2019.
Takwoingi 2015
Theart 2005
-
- Theart AC, Marais BJ, Gie RP, Hesseling AC, Beyers N. Criteria used for the diagnosis of childhood tuberculosis at primary health care level in a high-burden, urban setting. International Journal of Tuberculosis and Lung Disease 2005;9(11):1210-4. - PubMed
Wademan 2019
Whiting 2011
-
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. - PubMed
WHO 2014
-
- World Health Organization. Xpert MTB/RIF implementation manual. Technical and operational 'how-to' practical considerations. apps.who.int/iris/bitstream/10665/112469/1/9789241506700_eng.pdf 2014 (accessed prior to 29 April 2019).
WHO Consolidated Guidelines (Module 3) 2021
-
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detection, 2021 update. Licence: CC BY-NC-SA 3.0 IGO. who.int/publications/i/item/who-consolidated-guidelines-on-tuberculosis-... (accessed 12 October 2021).
WHO Consolidated Guidelines (Module 4) 2020
-
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment – drug-resistant tuberculosis treatment, June 2020. who.int/publications/i/item/9789240007048 (accessed 3 November 2021).
WHO Consolidated Guidelines (Module 5) 2022
-
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 5: management of tuberculosis in children and adolescents. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. www.who.int/publications/i/item/9789240046764 (accessed 29 April 2022).
WHO Global Tuberculosis Report 2021
-
- World Health Organization. Global tuberculosis report 2021. www.who.int/publications/digital/global-tuberculosis-report-2021 (accessed 18 October 2021).
WHO Operational handbook on tuberculosis 2021
-
- World Health Organization. WHO operational handbook on tuberculosis. Module 3: diagnosis - rapid diagnostics for tuberculosis detection, 2021 update. Licence: CC BY-NC-SA 3.0 IGO. www.who.int/publications/i/item/9789240030589 (accessed 2 November 2021).
Zar 2005
-
- Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet 2005;365(9454):130-4. - PubMed
Zar 2012
Zhang 2020
-
- Zhang M, Xue M, He JQ. Diagnostic accuracy of the new Xpert MTB/RIF Ultra for tuberculosis disease: a preliminary systematic review and meta-analysis. International Journal of Infectious Diseases 2020;90:35-45. - PubMed
Zifodya 2021
-
- Zifodya JS, Kreniske JS, Schiller I, Kohli M, Dendukuri N, Schumacher SG, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD009593. [DOI: 10.1002/14651858.CD009593.pub5] - DOI - PMC - PubMed
References to other published versions of this review
Kay 2020
-
- Kay AW, González Fernández L, Takwoingi Y, Eisenhut M, Vu RD, Steingart KR, Detjen AK, Mandalakas AM. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD013359. [DOI: 10.1002/14651858.CD013359] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

