A Multicenter Clinical Diagnostic Accuracy Study of SureStatus, an Affordable, WHO Emergency Use-Listed, Rapid, Point-Of-Care Antigen-Detecting Diagnostic Test for SARS-CoV-2
- PMID: 36066256
- PMCID: PMC9604065
- DOI: 10.1128/spectrum.01229-22
A Multicenter Clinical Diagnostic Accuracy Study of SureStatus, an Affordable, WHO Emergency Use-Listed, Rapid, Point-Of-Care Antigen-Detecting Diagnostic Test for SARS-CoV-2
Abstract
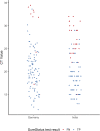
Access to reverse transcription-PCR (RT-PCR) testing, the gold standard for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection, is limited throughout the world, due to restricted resources, available infrastructure, and high costs. Antigen-detecting rapid diagnostic tests (Ag-RDTs) overcome some of these barriers, but independent clinical validations in settings of intended use are scarce. To inform the World Health Organization's (WHO) emergency use listing (EUL) procedure and ensure affordable, high-quality Ag-RDTs, we assessed the performance and ease of use of the SureStatus for SARS-CoV-2. For this prospective, multicenter diagnostic accuracy study, we recruited unvaccinated participants with presumed SARS-CoV-2 infection in India and Germany from December 2020 to March 2021, when the Alpha (B.1.1.7) variant was predominantly circulating. Paired swabs were performed for (i) routine clinical RT-PCR testing (sampling was either nasopharyngeal [NP] or combined NP and oropharyngeal [NP/OP]) and (ii) Ag-RDT (sampling was NP). Performance of the Ag-RDT was compared to RT-PCR overall and by predefined subgroups, e.g., cycle threshold (CT) value, symptoms, and days from symptom onset. To understand the usability, a system usability scale (SUS) questionnaire and ease-of-use (EoU) assessment were performed. A total of 1,119 participants were included in the analysis, of whom 205 (18.3%) were RT-PCR positive. SureStatus detected 169 out of 205 RT-PCR-positive participants, reporting a sensitivity of 82.4% (95% confidence interval [CI]: 76.6% to 87.1%) and a specificity of 98.5% (95% CI: 97.4% to 99.1%). In the first 7 days post-symptom onset, the sensitivity was 90.7% (95% CI: 83.5% to 94.9%), when CT values were low and viral loads were high. The test was characterized as easy to use (SUS, 85/100) and considered suitable for point-of-care settings, although quality concerns were raised due to visibly contaminated packaging of swabs included in the test kits. The SureStatus diagnostic test can be considered a reliable test during the first week of SARS-CoV-2 infection, with high sensitivity in combination with excellent usability. IMPORTANCE Our manufacturer-independent, prospective diagnostic accuracy study assessed clinical performance in participants presumed to have a SARS-CoV-2 infection at three study sites in two countries. We assessed the accuracy overall and in predefined subgroups (CT values and symptom duration). SureStatus performed with high sensitivity. Its sensitivity was particularly high in the first 3 days after symptom onset and when CT values were low (i.e., the viral load was high). The system usability and ease-of-use assessment complements the accuracy assessment of the test and highlights critical factors to facilitate the widespread use of SureStatus in point-of-care settings. The high sensitivity demonstrated by the evaluated Ag-RDT within the first days of symptoms, when most transmission occurs, supports the role of Ag-RDTs for public health-relevant screening. Evidence from this study was used to inform the World Health Organization Emergency Use Listing procedure.
Keywords: COVID-19; SARS-CoV-2; antigen-detecting rapid diagnostic tests; sensitivity; specificity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization. 6 October 2021. Antigen-detection in the diagnosis of SARS-CoV-2 infection. World Health Organization, Geneva, Switzerland. https://www.who.int/publications/i/item/antigen-detection-in-the-diagnos.... Accessed 30 March 2022.
-
- Denkinger CM, Brümmer LE, Katzenschlager S, Schmitz S. 31 August 2021. Diagnostics global health: rapid antigen tests for the diagnosis of a SARS-CoV-2 infection. Heidelberg, Germany. https://diagnosticsglobalhealth.org/. Accessed 30 March 2022.
-
- Brümmer LE, Katzenschlager S, Gaeddert M, Erdmann C, Schmitz S, Bota M, Grilli M, Larmann J, Weigand MA, Pollock NR, Macé A, Carmona S, Ongarello S, Sacks JA, Denkinger CM. 2021. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: a living systematic review and meta-analysis. PLoS Med 18:e1003735. doi: 10.1371/journal.pmed.1003735. - DOI - PMC - PubMed
-
- Foundation of Innovative New Diagnostics. 2022. About the ACT-Accelerator. Foundation of Innovative New Diagnostics, Geneva, Switzerland. https://www.finddx.org/covid-19/act-accelerator/. Accessed 30 March 2022.
-
- World Health Organization. 30 April 2021. WHO emergency use listing for in vitro diagnostics (IVDs) detecting SARS-CoV-2. World Health Organization, Geneva, Switzerland. https://extranet.who.int/pqweb/sites/default/files/documents/210430_EUL_.... Accessed 30 March 2022.
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

