Inhibition of EGFR Overcomes Acquired Lenvatinib Resistance Driven by STAT3-ABCB1 Signaling in Hepatocellular Carcinoma
- PMID: 36066408
- PMCID: PMC9574378
- DOI: 10.1158/0008-5472.CAN-21-4140
Inhibition of EGFR Overcomes Acquired Lenvatinib Resistance Driven by STAT3-ABCB1 Signaling in Hepatocellular Carcinoma
Abstract
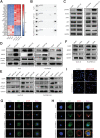
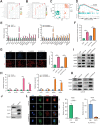
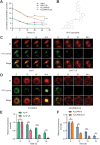
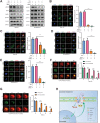
Lenvatinib is an inhibitor of multiple receptor tyrosine kinases that was recently authorized for first-line treatment of hepatocellular carcinoma (HCC). However, the clinical benefits derived from lenvatinib are limited, highlighting the urgent need to understand mechanisms of resistance. We report here that HCC cells develop resistance to lenvatinib by activating EGFR and stimulating the EGFR-STAT3-ABCB1 axis. Lenvatinib resistance was accompanied by aberrant cholesterol metabolism and lipid raft activation. ABCB1 was activated by EGFR in a lipid raft-dependent manner, which significantly enhanced the exocytosis of lenvatinib to mediate resistance. Furthermore, clinical specimens of HCC showed a correlation between the activation of the EGFR-STAT3-ABCB1 pathway and lenvatinib response. Erlotinib, an EGFR inhibitor that has also been shown to inhibit ABCB1, suppressed lenvatinib exocytosis, and combined treatment with lenvatinib and erlotinib demonstrated a significant synergistic effect on HCC both in vitro and in vivo. Taken together, these findings characterize a mechanism of resistance to a first-line treatment for HCC and offer a practical means to circumvent resistance and treat the disease.
Significance: HCC cells acquire resistance to lenvatinib by activating the EGFR-STAT3-ABCB1 pathway, identifying combined treatment with erlotinib as a strategy to overcome acquired resistance and improve the clinical benefit of lenvatinib.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
-
- Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018;15:599–616. - PubMed
-
- Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: a review in hepatocellular carcinoma. Drugs 2019;79:665–74. - PubMed
-
- Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res 2006;47:1597–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

