Challenges and Prospects in the Catalytic Conversion of Carbon Dioxide to Formaldehyde
- PMID: 36066469
- PMCID: PMC9827866
- DOI: 10.1002/anie.202204008
Challenges and Prospects in the Catalytic Conversion of Carbon Dioxide to Formaldehyde
Abstract
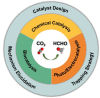
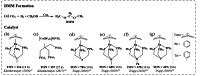
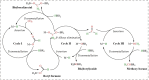
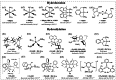
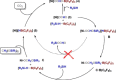
Formaldehyde (HCHO) is a crucial C1 building block for daily-life commodities in a wide range of industrial processes. Industrial production of HCHO today is based on energy- and cost-intensive gas-phase catalytic oxidation of methanol, which calls for exploring other and more sustainable ways of carrying out this process. Utilization of carbon dioxide (CO2 ) as precursor presents a promising strategy to simultaneously mitigate the carbon footprint and alleviate environmental issues. This Minireview summarizes recent progress in CO2 -to-HCHO conversion using hydrogenation, hydroboration/hydrosilylation as well as photochemical, electrochemical, photoelectrochemical, and enzymatic approaches. The active species, reaction intermediates, and mechanistic pathways are discussed to deepen the understanding of HCHO selectivity issues. Finally, shortcomings and prospects of the various strategies for sustainable reduction of CO2 to HCHO are discussed.
Keywords: Carbon Dioxide Hydrogenation; Formaldehyde Production; Hydroboration; Hydrosilylation; Photo/Electrochemistry.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Reuss G., Disteldorf W., Gamer A. O., Hilt A. in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000, pp. 735–768.
-
- Desmons S., Fauré R., Bontemps S., ACS Catal. 2019, 9, 9575–9588.
-
- Heim L. E., Konnerth H., Prechtl M. H. G., Green Chem. 2017, 19, 2347–2355.
-
- Waters T., O'Hair R. A. J., Wedd A. G., J. Am. Chem. Soc. 2003, 125, 3384–3396. - PubMed
-
- Kim T. H., Ramachandra B., Choi J. S., Saidutta M. B., Choo K. Y., Song S.-D., Rhee Y.-W., Catal. Lett. 2004, 98, 161–165.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

