Sterically and Electronically Flexible Pyridylidene Amine Dinitrogen Ligands at Palladium: Hemilabile cis/trans Coordination and Application in Dehydrogenation Catalysis
- PMID: 36066486
- PMCID: PMC10092520
- DOI: 10.1002/chem.202202672
Sterically and Electronically Flexible Pyridylidene Amine Dinitrogen Ligands at Palladium: Hemilabile cis/trans Coordination and Application in Dehydrogenation Catalysis
Abstract
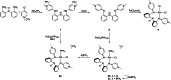
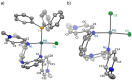
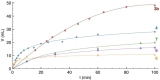
Ligand design is crucial for the development of new catalysts and materials with new properties. Herein, the synthesis and unique hemilabile coordination properties of new bis-pyridylidene amine (bis-PYE) ligands to palladium, and preliminary catalytic activity of these complexes in formic acid dehydrogenation are described. The synthetic pathway to form cationic complexes [Pd(bis-PYE)Cl(L)]X with a cis-coordinated N,N-bidentate bis-PYE ligand is flexible and provides access to a diversity of PdII complexes with different ancillary ligands (L=pyridine, DMAP, PPh3 , Cl, P(OMe)3 ). The 1 H NMR chemical shift of the trans-positioned PYE N-CH3 unit is identified as a convenient and diagnostic handle to probe the donor properties of these ancillary ligands and demonstrates the electronic flexibility of the PYE ligand sites. In the presence of a base, the originally cis-coordinated bis-PYE ligand adopts a N,N,N-tridentate coordination mode with the two PYE units in mutual trans position. This cis-trans isomerization is reverted in presence of an acid, demonstrating a unique structural and steric flexibility of the bis-PYE ligand at palladium in addition to its electronic adaptability. The palladium complexes are active in formic acid dehydrogenation to H2 and CO2 . The catalytic performance is directly dependent on the ligand bonding mode, the nature of the ancillary ligand, the counteranion, and additives. The most active system features a bidentate bis-PYE ligand, PPh3 as ancillary ligand and accomplishes turnover frequencies up to 525 h-1 in the first hour and turnover numbers of nearly 1000, which is the highest activity reported for palladium-based catalysts to date.
Keywords: cis/trans coordination; formic acid dehydrogenation; hemilability; palladium; pyridylidene-amines.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



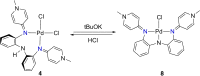


References
-
- Pombeiro A. J. L., Dalton Trans. 2019, 48, 13904–13906. - PubMed
-
- Shi Q., Thatcher R. J., Slattery J., Sauari P. S., Whitwood A. C., McGowan P. C., Douthwaite R. E., Chem. Eur. J. 2009, 15, 11346–11360. - PubMed
-
- Slattery J., Thatcher R. J., Shi Q., Douthwaite R. E., Pure Appl. Chem. 2010, 82, 1663–1671.
-
- Navarro M., Smith C. A., Albrecht M., Inorg. Chem. 2017, 56, 11688–11701. - PubMed
-
- Navarro M., Li M., Bernhard S., Albrecht M., Dalton Trans. 2018, 47, 659–662. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

