Medication adherence with denosumab in patients with bone metastases from solid tumors treated in routine clinical settings: a retrospective study
- PMID: 36066628
- PMCID: PMC9446633
- DOI: 10.1007/s00520-022-07333-7
Medication adherence with denosumab in patients with bone metastases from solid tumors treated in routine clinical settings: a retrospective study
Abstract
Purpose: To describe (non)adherence with denosumab among patients with solid tumors and bone metastases.
Methods: This retrospective, observational study pooled data from two completed prospective, multicenter cohort studies (X-TREME; Study 240) in adult patients with bone metastases from primary breast, prostate, lung, kidney, or other solid cancer types and administered denosumab 120 mg in routine clinical practice in Germany and Central and Eastern Europe. The studies were conducted between May 2012 and May 2017; pooled analysis was completed in August 2021. Medication adherence was described according to a three-component consensus taxonomy: initiation (first-ever administration ≤ 90 days from bone metastasis diagnosis), implementation (actual vs prescribed dosing; optimal implementation = regular/consistent dosing), and persistence (≤ 60-day gap between administrations at 3, 6, 9, and 12 months). Descriptive analyses were conducted for each cancer type.
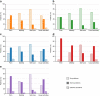
Results: The analysis included 1748 patients with solid tumors and bone metastases. Adherence with denosumab was generally high across the initiation, implementation, and persistence phases. Most patients experienced timely initiation (from 64.4% [kidney cancer] to 81.2% [breast cancer]) and optimal implementation (from 62.4% [lung cancer] to 72.5% [breast cancer]). The proportion of patients who were persistent with treatment at 6 months ranged from 41.4% (lung cancer) to 77.8% (prostate cancer).
Conclusions: This study revealed variations by cancer type in the initiation, implementation, and persistence of denosumab in patients with solid tumors and bone metastases in routine clinical practice. Further cancer-specific studies are warranted to examine the determinants of (non)adherence with denosumab, and potential ways to improve medication adherence.
Keywords: Denosumab; Implementation; Initiation; Medication adherence; Persistence; Real-world study.
© 2022. The Author(s).
Conflict of interest statement
IJD has received consulting fees for participation in advisory boards and has given several presentations at speaker bureaus for Amgen. RG has received honoraria from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Merck, MSD, Novartis, Roche, Sandoz, and Takeda; has participated in consulting or an advisory role for AbbVie, AstraZeneca, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Janssen, Merck, MSD, Novartis, Roche, and Takeda; has received research funding from Amgen, AstraZeneca, Bristol Myers Squibb, Celgene, Gilead, Merck, MSD, Novartis, Roche, Sandoz, and Takeda; and has received travel, accommodations and expenses from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Celgene Daiichi Sankyo, Gilead, Janssen, MSD, Novartis, and Roche. JJ has received consulting fees from AbbVie, Baxter, Bayer, Biotest, Bristol Myers Squibb, Celgene, Eli Lilly, Ipsen and Servier, Janssen-Cilag Ltd, Kedrion Biopharma, Merck Serono, Novartis, Octapharma, Pfizer, Pharma Mar, Puma Biotechnology, Roche, Sanofi, and Teva Pharmaceutical. CWK has participated in advisory boards for Amgen. BB is an employee of and holds stock options in Amgen. AA reports contract work with Amgen. AS is an employee of and holds stock options in Amgen. MK is a former employee of and holds stock options in Amgen. CJ is an employee of and holds stock options in Amgen. KB is a former employee of and holds stock options in Amgen. AT has no potential conflicts of interest to report. FH has participated in advisory boards for Amgen.
Figures

References
-
- Haslbauer F, Petzer A, Safanda M, Tomova A, Porubska M, Bajory Z, Niepel D, Jaeger C, Bjorklof K, Kalinin D, Greil R. Prospective observational study to evaluate the persistence of treatment with denosumab in patients with bone metastases from solid tumors in routine clinical practice: final analysis. Support Care Cancer. 2020;28:1855–1865. doi: 10.1007/s00520-019-04988-7. - DOI - PMC - PubMed
-
- European Medicines Agency (2011) Xgeva: summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/xgeva-epar-pr.... Accessed 24 Aug 2022
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

