The heterogeneity of microglial activation and its epigenetic and non-coding RNA regulations in the immunopathogenesis of neurodegenerative diseases
- PMID: 36066650
- PMCID: PMC11803019
- DOI: 10.1007/s00018-022-04536-3
The heterogeneity of microglial activation and its epigenetic and non-coding RNA regulations in the immunopathogenesis of neurodegenerative diseases
Abstract
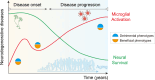
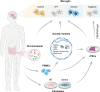
Microglia are resident immune cells in the brain and play a central role in the development and surveillance of the nervous system. Extensive gliosis is a common pathological feature of several neurodegenerative diseases, such as Alzheimer's disease (AD), the most common cause of dementia. Microglia can respond to multiple inflammatory insults and later transform into different phenotypes, such as pro- and anti-inflammatory phenotypes, thereby exerting different functions. In recent years, an increasing number of studies based on both traditional bulk sequencing and novel single-cell/nuclear sequencing and multi-omics analysis, have shown that microglial phenotypes are highly heterogeneous and dynamic, depending on the severity and stage of the disease as well as the particular inflammatory milieu. Thus, redirecting microglial activation to beneficial and neuroprotective phenotypes promises to halt the progression of neurodegenerative diseases. To this end, an increasing number of studies have focused on unraveling heterogeneous microglial phenotypes and their underlying molecular mechanisms, including those due to epigenetic and non-coding RNA modulations. In this review, we summarize the epigenetic mechanisms in the form of DNA and histone modifications, as well as the general non-coding RNA regulations that modulate microglial activation during immunopathogenesis of neurodegenerative diseases and discuss promising research approaches in the microglial era.
Keywords: DNA modification; Epigenetic regulation; Histone modification; Microglial activation; Microglial phenotypes; Neurodegenerative disease; Non-coding RNA; mRNA modification.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures



References
-
- Lane CA, Hardy J, Schott JM (2018) Alzheimer’s disease. Eur J Neurol 25(1):59–70. 10.1111/ene.13439 - PubMed
-
- Poewe W et al (2017) Parkinson disease. Nat Rev Dis Primers 3:17013. 10.1038/nrdp.2017.13 - PubMed
-
- Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 15(12):1257–1272. 10.1016/S1474-4422(16)30230-7 - PubMed
-
- Selkoe DJ (2012) Preventing Alzheimer’s disease. Science 337(6101):1488–1492. 10.1126/science.1228541 - PubMed
-
- Zaletel I et al (2018) Early impairments of hippocampal neurogenesis in 5xFAD mouse model of Alzheimer’s disease are associated with altered expression of SOXB transcription factors. J Alzheimers Dis 65(3):963–976. 10.3233/JAD-180277 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

