Potent anticancer activity of a novel iridium metallodrug via oncosis
- PMID: 36066676
- PMCID: PMC9448686
- DOI: 10.1007/s00018-022-04526-5
Potent anticancer activity of a novel iridium metallodrug via oncosis
Abstract
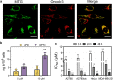
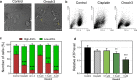
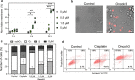
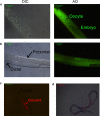
Oncosis (from Greek ónkos, meaning "swelling") is a non-apoptotic cell death process related to energy depletion. In contrast to apoptosis, which is the main form of cell death induced by anticancer drugs, oncosis has been relatively less explored but holds potential to overcome drug resistance phenomena. In this study, we report a novel rationally designed mitochondria-targeted iridium(III) complex (OncoIr3) with advantageous properties as a bioimaging agent. OncoIr3 exhibited potent anticancer activity in vitro against cancer cells and displayed low toxicity to normal dividing cells. Flow cytometry and fluorescence-based assays confirmed an apoptosis-independent mechanism involving energy depletion, mitochondrial dysfunction and cellular swelling that matched with the oncotic process. Furthermore, a Caenorhabditis elegans tumoral model was developed to test this compound in vivo, which allowed us to prove a strong oncosis-derived antitumor activity in animals (with a 41% reduction of tumor area). Indeed, OncoIr3 was non-toxic to the nematodes and extended their mean lifespan by 18%. Altogether, these findings might shed new light on the development of anticancer metallodrugs with non-conventional modes of action such as oncosis, which could be of particular interest for the treatment of apoptosis-resistant cancers.
Keywords: Anticancer agents; Antitumor activity; Caenorhabditis elegans; Iridium metallodrug; Oncosis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
Figures


References
-
- Ortega E, Vigueras G, Ballester FJ, Ruiz J. Targeting translation: a promising strategy for anticancer metallodrugs. Coord Chem Rev. 2021;446:214129. doi: 10.1016/j.ccr.2021.214129. - DOI
-
- Guan R, Xie L, Wang L, et al. Necroptosis-inducing iridium(III) complexes as regulators of cyclin-dependent kinases. Inorg Chem Front. 2021;8:1788–1794. doi: 10.1039/D0QI01430C. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

