Correlates of Dapivirine Vaginal Ring Acceptance among Women Participating in an Open Label Extension Trial
- PMID: 36066762
- PMCID: PMC10102709
- DOI: 10.1007/s10461-022-03841-z
Correlates of Dapivirine Vaginal Ring Acceptance among Women Participating in an Open Label Extension Trial
Abstract
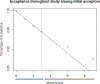
MTN-025/HOPE was an open-label trial of the dapivirine vaginal ring conducted in four African countries between 2016 and 2018. Women were first offered one ring monthly (at baseline, months 1 and 2), thereafter, transitioned to a more applicable real-world dispensation schedule, - 3 rings quarterly (at months 3, 6 and 9). Logistic regression analysis was used to assess correlates of ring acceptance at baseline and through follow-up. A total of 1456 women (median age 31 years) enrolled, 1342 (92.2%) accepted the ring at baseline and 1163 (79.9%) accepted the ring(s) at all visits. Changing ring dispensation from a monthly to a quarterly schedule had no negative effect on acceptance. Having a primary partner and him knowing about the ring being offered in HOPE, use of long-acting contraception (implants, injections, IUDs) or sterilization were associated with ring acceptance, along with prior strong intention to use the ring in the future. Efforts should consider these factors when rolling out the ring for HIV prevention.
Keywords: African women; HIV Pre-Exposure Prophylaxis; acceptance; choice; dapivirine vaginal ring; microbicide.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Declarations
Figures

References
-
- UNAIDS. Women and HIV. A spotlight on adolescent girls and young women. 2019.
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI069423/AI/NIAID NIH HHS/United States
- P30 MH062246/MH/NIMH NIH HHS/United States
- UM1 AI068633/AI/NIAID NIH HHS/United States
- U01 AI068633/AI/NIAID NIH HHS/United States
- UM1 AI068615/AI/NIAID NIH HHS/United States
- UM1 AI106707/AI/NIAID NIH HHS/United States
- UM1 AI069469/AI/NIAID NIH HHS/United States
- UM1 AI069518/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI069436/AI/NIAID NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
- U01 AI069436/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

