Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial
- PMID: 36066977
- PMCID: PMC9675448
- DOI: 10.1172/jci.insight.159863
Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial
Abstract
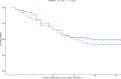
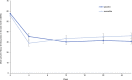
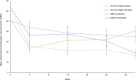
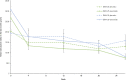
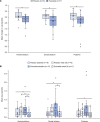
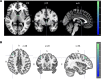
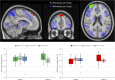
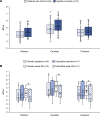
BackgroundAlcohol use disorder (AUD) is a chronic, relapsing brain disorder that accounts for 5% of deaths annually, and there is an urgent need to develop new targets for therapeutic intervention. The glucagon-like peptide-1 (GLP-1) receptor agonist exenatide reduces alcohol consumption in rodents and nonhuman primates, but its efficacy in patients with AUD is unknown.MethodsIn a randomized, double-blinded, placebo-controlled clinical trial, treatment-seeking AUD patients were assigned to receive exenatide (2 mg subcutaneously) or placebo once weekly for 26 weeks, in addition to standard cognitive-behavioral therapy. The primary outcome was reduction in number of heavy drinking days. A subgroup also completed functional MRI (fMRI) and single-photon emission CT (SPECT) brain scans.ResultsA total of 127 patients were enrolled. Our data revealed that although exenatide did not significantly reduce the number of heavy drinking days compared with placebo, it significantly attenuated fMRI alcohol cue reactivity in the ventral striatum and septal area, which are crucial brain areas for drug reward and addiction. In addition, dopamine transporter availability was lower in the exenatide group compared with the placebo group. Exploratory analyses revealed that exenatide significantly reduced heavy drinking days and total alcohol intake in a subgroup of obese patients (BMI > 30 kg/m2). Adverse events were mainly gastrointestinal.ConclusionThis randomized controlled trial on the effects of a GLP-1 receptor agonist in AUD patients provides new important knowledge on the effects of GLP-1 receptor agonists as a novel treatment target in addiction.Trial registrationEudraCT: 2016-003343-11. ClinicalTrials.gov (NCT03232112).FundingNovavi Foundation; Research Foundation, Mental Health Services, Capital Region of Denmark; Research Foundation, Capital Region of Denmark; Ivan Nielsen Foundation; A.P. Moeller Foundation; Augustinus Foundation; Woerzner Foundation; Grosserer L.F. Foghts Foundation; Hartmann Foundation; Aase and Ejnar Danielsen Foundation; P.A. Messerschmidt and Wife Foundation; and Lundbeck Foundation.
Keywords: Addiction; Clinical Trials; Neuroimaging; Neuroscience; Pharmacology.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

