Resensitization in suspected penicillin allergy
- PMID: 36067012
- PMCID: PMC10087608
- DOI: 10.1111/all.15508
Resensitization in suspected penicillin allergy
Abstract
Background: The diagnosis of allergic reactions to penicillins (AR-PEN) is very complex as there is a loss of sensitization over time, which leads to negative skin tests (STs) and specific IgE in serum, and even to tolerance to the drug involved. However, STs may become positive after subsequent exposure to the culprit drug (resensitization), with the risk of inducing potentially severe reactions. The exact rate of resensitization to penicillins is unknown, ranging from 0% to 27.9% in published studies.
Objectives: To analyze the rate of resensitization in patients with suggestive AR-PEN by repeating STs (retest) after an initial evaluation (IE).
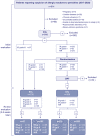
Material and methods: Patients with suspected AR-PEN were prospectively evaluated between 2017 and 2020. They underwent STs, and a randomized group also underwent a drug provocation test (DPT) with the culprit. Only patients with negative STs and/or DPT were included. All included cases were retested by STs at 2-8 weeks.
Results: A total of 545 patients were included: 296 reporting immediate reactions (IRs) and 249 non-immediate reactions (NIRs). Eighty (14.7%) cases had positive results in retest (RT+): 63 (21.3%) IRs and 17 (6.8%) NIRs (p < 0.0001). The rate of RT+ was higher in anaphylaxis compared with all other reactions (45.8% vs 9.1%, p < 0.0001). The risk of RT+ was higher from the fifth week after IE (OR: 4.64, CI: 2.1-11.6; p < 0.001) and increased with the patient's age (OR: 1.02; CI: 1.01-1.04; p = 0.009).
Conclusions: Due to the high rate of resensitization, retest should be included in the diagnostic algorithm of IRs to penicillins after an initial negative study, especially in anaphylaxis, to avoid potentially severe reactions after subsequent prescriptions of these drugs.
Keywords: anaphylaxis; drug provocation test; penicillins; resensitization; skin test, specific IgE.
© 2022 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest. All authors have given final approval to the version to be published. Research is part of their daily activities. All the authors had full access to all the data and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Figures
References
-
- Doña I, Blanca‐López N, Torres MJ, et al. Drug hypersensitivity reactions: response patterns, drug involved, and temporal variations in a large series of patients. J Investig Allergol Clin Immunol. 2012;22(5):363‐371. - PubMed
-
- Romano A et al. Towards a more precise diagnosis of hypersensitivity to beta‐lactams–an EAACI position paper. Allergy. 2020;75(6):1300‐1315. - PubMed
-
- Torres MJ, Blanca M, Fernandez J, et al. Diagnosis of immediate allergic reactions to beta‐lactam antibiotics. Allergy. 2003;58(10):961‐972. - PubMed
-
- Brockow K, Ardern‐Jones MR, Mockenhaupt M, et al. EAACI position paper on how to classify cutaneous manifestations of drug hypersensitivity. Allergy. 2019;74(1):14‐27. - PubMed
-
- Moreno E, Laffond E, Muñoz‐Bellido F, et al. Performance in real life of the European network on drug allergy algorithm in immediate reactions to beta‐lactam antibiotics. Allergy. 2016;71(12):1787‐1790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials