Mechanisms regulating neutrophil responses in immunity, allergy, and autoimmunity
- PMID: 36067034
- PMCID: PMC10087481
- DOI: 10.1111/all.15505
Mechanisms regulating neutrophil responses in immunity, allergy, and autoimmunity
Abstract
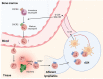
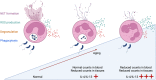
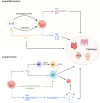
Neutrophil granulocytes, or neutrophils, are the most abundant circulating leukocytes in humans and indispensable for antimicrobial immunity, as exemplified in patients with inborn and acquired defects of neutrophils. Neutrophils were long regarded as the foot soldiers of the immune system, solely destined to execute a set of effector functions against invading pathogens before undergoing apoptosis, the latter of which was ascribed to their short life span. This simplistic understanding of neutrophils has now been revised on the basis of insights gained from the use of mouse models and single-cell high-throughput techniques, revealing tissue- and context-specific roles of neutrophils in guiding immune responses. These studies also demonstrated that neutrophil responses were controlled by sophisticated feedback mechanisms, including directed chemotaxis of neutrophils to tissue-draining lymph nodes resulting in modulation of antimicrobial immunity and inflammation. Moreover, findings in mice and humans showed that neutrophil responses adapted to different deterministic cytokine signals, which controlled their migration and effector function as well as, notably, their biologic clock by affecting the kinetics of their aging. These mechanistic insights have important implications for health and disease in humans, particularly, in allergic diseases, such as atopic dermatitis and allergic asthma bronchiale, as well as in autoinflammatory and autoimmune diseases. Hence, our improved understanding of neutrophils sheds light on novel therapeutic avenues, focusing on molecularly defined biologic agents.
Keywords: autoimmunity; autoinflammation; immunodeficiency; infection; inflammation.
© 2022 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interests related to this manuscript.
Figures

References
-
- Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The neutrophil. Immunity. 2021;54:1377‐1391. - PubMed
-
- Leiding JW, Holland SM. Chronic granulomatous disease. GeneReviews®. Published Online First: 21 April 2022. https://www.ncbi.nlm.nih.gov/books/NBK99496/
-
- Rood JE, Behrens EM. Inherited autoinflammatory syndromes. Annu Rev Pathol Mech Dis. 2021;17:227‐249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

