Strongyloides stercoralis infection induces gut dysbiosis in chronic kidney disease patients
- PMID: 36067216
- PMCID: PMC9481163
- DOI: 10.1371/journal.pntd.0010302
Strongyloides stercoralis infection induces gut dysbiosis in chronic kidney disease patients
Abstract
Background: Strongyloides stercoralis infection typically causes severe symptoms in immunocompromised patients. This infection can also alter the gut microbiota and is often found in areas where chronic kidney disease (CKD) is common. However, the relationship between S. stercoralis and the gut microbiome in chronic kidney disease (CKD) is not understood fully. Recent studies have shown that gut dysbiosis plays an important role in the progression of CKD. Hence, this study aims to investigate the association of S. stercoralis infection and gut microbiome in CKD patients.
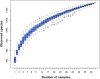
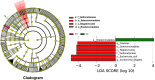
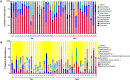
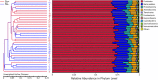
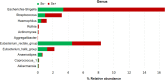
Methodology/principal findings: Among 838 volunteers from Khon Kaen Province, northeastern Thailand, 40 subjects with CKD were enrolled and divided into two groups (S. stercoralis-infected and -uninfected) matched for age, sex and biochemical parameters. Next-generation technology was used to amplify and sequence the V3-V4 region of the 16S rRNA gene to provide a profile of the gut microbiota. Results revealed that members of the S. stercoralis-infected group had lower gut microbial diversity than was seen in the uninfected group. Interestingly, there was significantly greater representation of some pathogenic bacteria in the S. stercoralis-infected CKD group, including Escherichia-Shigella (P = 0.013), Rothia (P = 0.013) and Aggregatibacter (P = 0.03). There was also a trend towards increased Actinomyces, Streptococcus and Haemophilus (P > 0.05) in this group. On the other hand, the S. stercoralis-infected CKD group had significantly lower representation of SCFA-producing bacteria such as Anaerostipes (P = 0.01), Coprococcus_1 (0.043) and a non-significant decrease of Akkermansia, Eubacterium rectale and Eubacterium hallii (P > 0.05) relative to the uninfected group. Interesting, the genera Escherichia-Shigella and Anaerostipes exhibited opposing trends, which were significantly related to sex, age, infection status and CKD stages. The genus Escherichia-Shigella was significantly more abundant in CKD patients over the age of 65 years and infected with S. stercoralis. A correlation analysis showed inverse moderate correlation between the abundance of the genus of Escherichia-Shigella and the level of estimated glomerular filtration rate (eGFR).
Conclusions/significance: Conclusion, the results suggest that S. stercoralis infection induced gut dysbiosis in the CKD patients, which might be involved in CKD progression.
Conflict of interest statement
The authors have declared that no competing interest exist.
Figures


Similar articles
-
Strongyloides stercoralis infection reduces Fusicatenibacter and Anaerostipes in the gut and increases bacterial amino-acid metabolism in early-stage chronic kidney disease.Heliyon. 2023 Sep 6;9(9):e19859. doi: 10.1016/j.heliyon.2023.e19859. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809389 Free PMC article.
-
Gut microbiota diversity in human strongyloidiasis differs little in two different regions in endemic areas of Thailand.PLoS One. 2022 Dec 30;17(12):e0279766. doi: 10.1371/journal.pone.0279766. eCollection 2022. PLoS One. 2022. PMID: 36584127 Free PMC article.
-
Alterations to the Gut Microbiota and Their Correlation With Inflammatory Factors in Chronic Kidney Disease.Front Cell Infect Microbiol. 2019 Jun 12;9:206. doi: 10.3389/fcimb.2019.00206. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31245306 Free PMC article.
-
Specific alterations in gut microbiota in patients with chronic kidney disease: an updated systematic review.Ren Fail. 2021 Dec;43(1):102-112. doi: 10.1080/0886022X.2020.1864404. Ren Fail. 2021. PMID: 33406960 Free PMC article.
-
The clinical impact of gut microbiota in chronic kidney disease.Korean J Intern Med. 2020 Nov;35(6):1305-1316. doi: 10.3904/kjim.2020.411. Epub 2020 Sep 29. Korean J Intern Med. 2020. PMID: 32872729 Free PMC article. Review.
Cited by
-
Strongyloides stercoralis infection reduces Fusicatenibacter and Anaerostipes in the gut and increases bacterial amino-acid metabolism in early-stage chronic kidney disease.Heliyon. 2023 Sep 6;9(9):e19859. doi: 10.1016/j.heliyon.2023.e19859. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809389 Free PMC article.
-
Evaluation of the Correlation between Gut Microbiota and Renal Function in Chronic Kidney Disease Patients.J Microbiol Biotechnol. 2025 Jun 17;35:e2502039. doi: 10.4014/jmb.2502.02039. J Microbiol Biotechnol. 2025. PMID: 40537901 Free PMC article.
-
Slight Changes in the Gut Microbiome in Early-stage Chronic Kidney Disease of Unknown Etiology.Microbes Environ. 2023;38(3):ME22097. doi: 10.1264/jsme2.ME22097. Microbes Environ. 2023. PMID: 37635077 Free PMC article.
-
Combined effect of microbially derived cecal SCFA and host genetics on feed efficiency in broiler chickens.Microbiome. 2023 Sep 1;11(1):198. doi: 10.1186/s40168-023-01627-6. Microbiome. 2023. PMID: 37653442 Free PMC article.
-
Monks: A Population at Risk for Liver Fluke and Skin-Penetrating Helminths.Trop Med Infect Dis. 2023 Feb 23;8(3):135. doi: 10.3390/tropicalmed8030135. Trop Med Infect Dis. 2023. PMID: 36977136 Free PMC article.
References
-
- Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron. 1996;74(2):349–55. Epub 1996/01/01. doi: 10.1159/000189334 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

