Spatiotemporal analysis of tumour-infiltrating immune cells in biliary carcinogenesis
- PMID: 36068277
- PMCID: PMC9596479
- DOI: 10.1038/s41416-022-01933-0
Spatiotemporal analysis of tumour-infiltrating immune cells in biliary carcinogenesis
Abstract
Background: Intraductal papillary neoplasms (IPN) and biliary epithelial neoplasia (BilIN) are well-defined precursor lesions of biliary tract carcinoma (BTC). The aim of this study was to provide a comprehensive characterisation of the inflammatory microenvironment in BTC precursor lesions.
Methods: Immunohistochemistry was employed to assess tumour-infiltrating immune cells in tissue samples from patients, for whom precursor lesions were identified alongside invasive BTC. The spatiotemporal evolution of the immune microenvironment during IPN-associated carcinogenesis was comprehensively analysed using triplet sample sets of non-neoplastic epithelium, precursor lesion and invasive BTC. Immune-cell dynamics during IPN- and BilIN-associated carcinogenesis were subsequently compared.
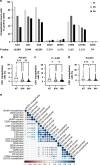
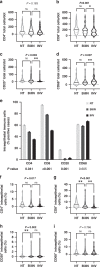
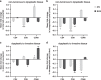
Results: Stromal CD3+ (P = 0.002), CD4+ (P = 0.007) and CD8+ (P < 0.001) T cells, CD20+ B cells (P = 0.008), MUM1+ plasma cells (P = 0.012) and CD163+ M2-like macrophages (P = 0.008) significantly decreased in IPN compared to non-tumorous biliary epithelium. Upon transition from IPN to invasive BTC, stromal CD68+ (P = 0.001) and CD163+ (P < 0.001) macrophages significantly increased. In contrast, BilIN-driven carcinogenesis was characterised by significant reduction of intraepithelial CD8+ T-lymphocytic infiltration from non-tumorous epithelium via BilIN (P = 0.008) to BTC (P = 0.004).
Conclusion: IPN and BilIN are immunologically distinct entities that undergo different immune-cell variations during biliary carcinogenesis. Intraepithelial CD8+ T-lymphocytic infiltration of biliary tissue decreased already at the IPN-precursor stage, whereas BilIN-associated carcinogenesis showed a slowly progressing reduction towards invasive carcinoma.
© 2022. The Author(s).
Conflict of interest statement
PS: grant, boards and presentations from Novartis, and boards from Incyte. BG: advisory board from Novartis.
Figures


References
-
- Brierley JD, Gospodarowicz MK, Wittekind C. (eds). TNM classification of malignant tumours. 8th edn. Hoboken, NJ, USA: Wiley-Blackwell; 2016. p. 272.
-
- Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th edn. Cham, Switzerland: Springer International Publishing; 2017. p. 1032.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

