Tachycardiomyopathy entails a dysfunctional pattern of interrelated mitochondrial functions
- PMID: 36068416
- PMCID: PMC9448689
- DOI: 10.1007/s00395-022-00949-0
Tachycardiomyopathy entails a dysfunctional pattern of interrelated mitochondrial functions
Abstract
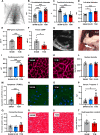
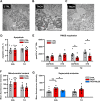
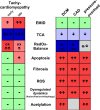
Tachycardiomyopathy is characterised by reversible left ventricular dysfunction, provoked by rapid ventricular rate. While the knowledge of mitochondria advanced in most cardiomyopathies, mitochondrial functions await elucidation in tachycardiomyopathy. Pacemakers were implanted in 61 rabbits. Tachypacing was performed with 330 bpm for 10 days (n = 11, early left ventricular dysfunction) or with up to 380 bpm over 30 days (n = 24, tachycardiomyopathy, TCM). In n = 26, pacemakers remained inactive (SHAM). Left ventricular tissue was subjected to respirometry, metabolomics and acetylomics. Results were assessed for translational relevance using a human-based model: induced pluripotent stem cell derived cardiomyocytes underwent field stimulation for 7 days (TACH-iPSC-CM). TCM animals showed systolic dysfunction compared to SHAM (fractional shortening 37.8 ± 1.0% vs. 21.9 ± 1.2%, SHAM vs. TCM, p < 0.0001). Histology revealed cardiomyocyte hypertrophy (cross-sectional area 393.2 ± 14.5 µm2 vs. 538.9 ± 23.8 µm2, p < 0.001) without fibrosis. Mitochondria were shifted to the intercalated discs and enlarged. Mitochondrial membrane potential remained stable in TCM. The metabolite profiles of ELVD and TCM were characterised by profound depletion of tricarboxylic acid cycle intermediates. Redox balance was shifted towards a more oxidised state (ratio of reduced to oxidised nicotinamide adenine dinucleotide 10.5 ± 2.1 vs. 4.0 ± 0.8, p < 0.01). The mitochondrial acetylome remained largely unchanged. Neither TCM nor TACH-iPSC-CM showed relevantly increased levels of reactive oxygen species. Oxidative phosphorylation capacity of TCM decreased modestly in skinned fibres (168.9 ± 11.2 vs. 124.6 ± 11.45 pmol·O2·s-1·mg-1 tissue, p < 0.05), but it did not in isolated mitochondria. The pattern of mitochondrial dysfunctions detected in two models of tachycardiomyopathy diverges from previously published characteristic signs of other heart failure aetiologies.
Keywords: Acetylome; Mitochondria; Redox; Tachycardiomyopathy.
© 2022. The Author(s).
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures




References
-
- Abbate A, Biondi-Zoccai GGL, Bussani R, Dobrina A, Camilot D, Feroce F, Rossiello R, Baldi F, Silvestri F, Biasucci LM, Baldi A. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J Am Coll Cardiol. 2003;41:753–760. doi: 10.1016/S0735-1097(02)02959-5. - DOI - PubMed
-
- Agnetti G, Kaludercic N, Kane LA, Elliott ST, Guo Y, Chakir K, Samantapudi D, Paolocci N, Tomaselli GF, Kass DA, van Eyk JE. Modulation of mitochondrial proteome and improved mitochondrial function by biventricular pacing of dyssynchronous failing hearts. Circ Cardiovasc Genet. 2010;3:78–87. doi: 10.1161/CIRCGENETICS.109.871236. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

