High-Dose Intravenous Iron with Either Ferric Carboxymaltose or Ferric Derisomaltose: A Benefit-Risk Assessment
- PMID: 36068430
- PMCID: PMC9492608
- DOI: 10.1007/s40264-022-01216-w
High-Dose Intravenous Iron with Either Ferric Carboxymaltose or Ferric Derisomaltose: A Benefit-Risk Assessment
Abstract
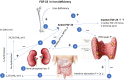
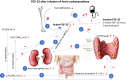
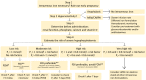
The intravenous iron formulations ferric carboxymaltose (FCM) and ferric derisomaltose (FDI) offer the possibility of administering a large amount of iron in one infusion. This results in faster correction of anemia and the formulations being better tolerated than oral iron formulations. This triad of logistic advantages, improved patient convenience, and fast correction of anemia explains the fact that intravenous iron formulations nowadays are frequently prescribed worldwide in the treatment of iron deficiency anemia. However, these formulations may result in hypophosphatemia by inducing a strong increase in active fibroblast growth factor-23 (FGF-23), a hormone that stimulates renal phosphate excretion. This effect is much more pronounced with FCM than with FDI, and therefore the risk of developing hypophosphatemia is remarkably higher with FCM than with FDI. Repeated use of FCM may result in severe osteomalacia, which is characterized by bone pain, Looser zones (pseudofractures), and low-trauma fractures. Intravenous iron preparations are also associated with other adverse effects, of which hypersensitivity reactions are the most important and are usually the result of a non-allergic complement activation on nanoparticles of free labile iron-Complement Activation-Related Pseudo-Allergy (CARPA). The risk on these hypersensitivity reactions can be reduced by choosing a slow infusion rate. Severe hypersensitivity reactions were reported in < 1% of prospective trials and the incidence seems comparable between the two formulations. A practical guideline has been developed based on baseline serum phosphate concentrations and predisposing risk factors, derived from published cases and risk factor analyses from trials, in order to establish the safe use of these formulations.
© 2022. The Author(s).
Conflict of interest statement
Johannes M.M. Boots received lecture fees and participated in advisory boards from Cablon Medical, The Netherlands; Pharmacosmos, Denmark; and Vifor Pharma, The Netherlands. Rogier A.M. Quax has no disclosures with regard to this manuscript.
Figures
References
-
- Müller A. Classification and properties of iron preparations. Arzneimittelforschung (Drug Res). 1974;24:880–883. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

