Effects of a mindfulness-based versus a health self-management intervention on objective cognitive performance in older adults with subjective cognitive decline (SCD): a secondary analysis of the SCD-Well randomized controlled trial
- PMID: 36068621
- PMCID: PMC9446839
- DOI: 10.1186/s13195-022-01057-w
Effects of a mindfulness-based versus a health self-management intervention on objective cognitive performance in older adults with subjective cognitive decline (SCD): a secondary analysis of the SCD-Well randomized controlled trial
Abstract
Background: Older individuals with subjective cognitive decline (SCD) perceive that their cognition has declined but do not show objective impairment on neuropsychological tests. Individuals with SCD are at elevated risk of objective cognitive decline and incident dementia. Non-pharmacological interventions (including mindfulness-based and health self-management approaches) are a potential strategy to maintain or improve cognition in SCD, which may ultimately reduce dementia risk.
Methods: This study utilized data from the SCD-Well randomized controlled trial. One hundred forty-seven older adults with SCD (MAge = 72.7 years; 64% female) were recruited from memory clinics in four European countries and randomized to one of two group-based, 8-week interventions: a Caring Mindfulness-based Approach for Seniors (CMBAS) or a health self-management program (HSMP). Participants were assessed at baseline, post-intervention (week 8), and at 6-month follow-up (week 24) using a range of cognitive tests. From these tests, three composites were derived-an "abridged" Preclinical Alzheimer's Cognitive Composite 5 (PACC5Abridged), an attention composite, and an executive function composite. Both per-protocol and intention-to-treat analyses were performed. Linear mixed models evaluated the change in outcomes between and within arms and adjusted for covariates and cognitive retest effects. Sensitivity models repeated the per-protocol analyses for participants who attended ≥ 4 intervention sessions.
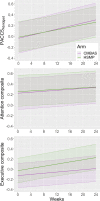
Results: Across all cognitive composites, there were no significant time-by-trial arm interactions and no measurable cognitive retest effects; sensitivity analyses supported these results. Improvements, however, were observed within both trial arms on the PACC5Abridged from baseline to follow-up (Δ [95% confidence interval]: CMBAS = 0.34 [0.19, 0.48]; HSMP = 0.30 [0.15, 0.44]). There was weaker evidence of an improvement in attention but no effects on executive function.
Conclusions: Two non-pharmacological interventions conferred small, non-differing improvements to a global cognitive composite sensitive to amyloid-beta-related decline. There was weaker evidence of an effect on attention, and no evidence of an effect on executive function. Importantly, observed improvements were maintained beyond the end of the interventions. Improving cognition is an important step toward dementia prevention, and future research is needed to delineate the mechanisms of action of these interventions and to utilize clinical endpoints (i.e., progression to mild cognitive impairment or dementia).
Trial registration: ClinicalTrials.gov, NCT03005652.
Keywords: Cognition; Compassion; Mindfulness; Randomized controlled trial; Subjective cognitive decline.
© 2022. The Author(s).
Conflict of interest statement
T.B. has received honoraria for workshops on MBIs and is the co-author of a book on mindfulness-based cognitive therapy. The other authors declare that they have no competing interests.
Figures
References
-
- Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. - DOI - PMC - PubMed
-
- Slot RER, Sikkes SAM, Berkhof J, Brodaty H, Buckley R, Cavedo E, Dardiotis E, Guillo-Benarous F, Hampel H, Kochan NA, et al. Subjective cognitive decline and rates of incident Alzheimer’s disease and non-Alzheimer’s disease dementia. Alzheimers Dement. 2019;15:465–476. doi: 10.1016/j.jalz.2018.10.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

