CircPRKCH modulates extracellular matrix formation and metabolism by regulating the miR-145/HGF axis in osteoarthritis
- PMID: 36068644
- PMCID: PMC9447342
- DOI: 10.1186/s13075-022-02893-9
CircPRKCH modulates extracellular matrix formation and metabolism by regulating the miR-145/HGF axis in osteoarthritis
Abstract
Background: Osteoarthritis (OA) is a chronic degenerative joint disease. Extracellular matrix (ECM) degradation is essential for OA progression. Previous studies have shown that circular RNAs (circRNAs) are involved in the pathological process of OA. CircPRKCH has been shown to be upregulated in OA chondrocytes. The present study was aimed to explore the roles of circPRKCH in vivo and in vitro models of OA and its underlying molecular mechanisms.
Methods: IL-1β-induced chondrocytes and mice injected with monosodium iodoacetate were used as OA models in vitro and in vivo, respectively. RT-qPCR was performed to measure the expression of circPRKCH, miR-145, and HGF in cartilage tissues and chondrocytes. The interaction between miR-145 and circPRKCH or HGF was verified by a dual-luciferase reporter assay. Chondrocyte apoptosis, viability, and ECM-related proteins were examined by flow cytometry, MTT assay, and Western blotting, respectively. Histopathological changes were detected by HE and Safranin O-fast green staining.
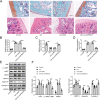
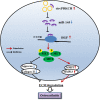
Results: The expression of circPRKCH and HGF was increased in OA cartilage tissues and IL-1β-treated chondrocytes, while miR-145 expression was decreased. IL-1β induced chondrocyte apoptosis and ECM degradation in chondrocytes. Moreover, circPRKCH promoted HGF expression and activated HGF/c-MET by directly binding to miR-145. miR-145 knockdown or HGF overexpression significantly reversed circPRKCH knockdown-mediated inhibition of apoptosis and ECM degradation in IL-1β-induced chondrocytes. Besides, miR-145 overexpression alleviated IL-1β-induced chondrocyte apoptosis and ECM degradation by inhibiting HGF/c-MET. Finally, circPRKCH knockdown reduced ECM degradation by regulating the miR-145/HGF axis in an experimental OA model in mice.
Conclusion: Our study demonstrated that circPRKCH promoted chondrocyte apoptosis and ECM degradation via the miR-145/HGF axis in OA, which may provide a novel target for OA treatment.
Keywords: CircPRKCH; Extracellular matrix; Hepatocyte growth factor; Osteoarthritis; miR-145.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Circ_DHRS3 positively regulates GREM1 expression by competitively targeting miR-183-5p to modulate IL-1β-administered chondrocyte proliferation, apoptosis and ECM degradation.Int Immunopharmacol. 2021 Feb;91:107293. doi: 10.1016/j.intimp.2020.107293. Epub 2020 Dec 23. Int Immunopharmacol. 2021. PMID: 33360372
-
Circ_0110251 overexpression alleviates IL-1β-induced chondrocyte apoptosis and extracellular matrix degradation by regulating miR-3189-3p/SPRY1 axis in osteoarthritis.Autoimmunity. 2022 May;55(3):168-178. doi: 10.1080/08916934.2022.2027917. Epub 2022 Feb 24. Autoimmunity. 2022. PMID: 35196925
-
Circ_0134111 knockdown relieves IL-1β-induced apoptosis, inflammation and extracellular matrix degradation in human chondrocytes through the circ_0134111-miR-515-5p-SOCS1 network.Int Immunopharmacol. 2021 Jun;95:107495. doi: 10.1016/j.intimp.2021.107495. Epub 2021 Mar 5. Int Immunopharmacol. 2021. PMID: 33684877
-
LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis.Cell Biosci. 2019 Jul 1;9:54. doi: 10.1186/s13578-019-0302-2. eCollection 2019. Cell Biosci. 2019. PMID: 31304004 Free PMC article. Review.
-
The role of apoptosis in the pathogenesis of osteoarthritis.Int Orthop. 2023 Aug;47(8):1895-1919. doi: 10.1007/s00264-023-05847-1. Epub 2023 Jun 9. Int Orthop. 2023. PMID: 37294429 Review.
Cited by
-
Causal influences of osteoarthritis on COVID-19: a Mendelian randomization study.Front Med (Lausanne). 2023 Oct 31;10:1287043. doi: 10.3389/fmed.2023.1287043. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38020136 Free PMC article.
-
Molecular roles in membrane receptor signaling pathways and cascade reactions in chondrocytes: a review.J Mol Histol. 2025 Feb 23;56(2):94. doi: 10.1007/s10735-025-10368-9. J Mol Histol. 2025. PMID: 39988650 Review.
-
Recent progress in exosomal non-coding RNAs research related to idiopathic pulmonary fibrosis.Front Genet. 2025 Mar 27;16:1556495. doi: 10.3389/fgene.2025.1556495. eCollection 2025. Front Genet. 2025. PMID: 40212286 Free PMC article. Review.
-
CircRNAs in osteoarthritis: research status and prospect.Front Genet. 2023 May 9;14:1173812. doi: 10.3389/fgene.2023.1173812. eCollection 2023. Front Genet. 2023. PMID: 37229197 Free PMC article. Review.
-
Effects and action mechanisms of individual cytokines contained in PRP on osteoarthritis.J Orthop Surg Res. 2023 Sep 22;18(1):713. doi: 10.1186/s13018-023-04119-3. J Orthop Surg Res. 2023. PMID: 37735688 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

