Multisensory integration in neurons of the medial pulvinar of macaque monkey
- PMID: 36068947
- PMCID: PMC10110443
- DOI: 10.1093/cercor/bhac337
Multisensory integration in neurons of the medial pulvinar of macaque monkey
Abstract
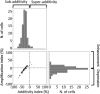
The pulvinar is a heterogeneous thalamic nucleus, which is well developed in primates. One of its subdivisions, the medial pulvinar, is connected to many cortical areas, including the visual, auditory, and somatosensory cortices, as well as with multisensory areas and premotor areas. However, except for the visual modality, little is known about its sensory functions. A hypothesis is that, as a region of convergence of information from different sensory modalities, the medial pulvinar plays a role in multisensory integration. To test this hypothesis, 2 macaque monkeys were trained to a fixation task and the responses of single-units to visual, auditory, and auditory-visual stimuli were examined. Analysis revealed auditory, visual, and multisensory neurons in the medial pulvinar. It also revealed multisensory integration in this structure, mainly suppressive (the audiovisual response is less than the strongest unisensory response) and subadditive (the audiovisual response is less than the sum of the auditory and the visual responses). These findings suggest that the medial pulvinar is involved in multisensory integration.
Keywords: audiovisual; medial pulvinar; multisensory; single-units.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permission@oup.com.
Figures








References
-
- Allman BL, Meredith MA. Multisensory processing in “unimodal” neurons: cross-modal subthreshold auditory effects in cat Extrastriate visual cortex. J Neurophysiol. 2007:98(1):545–549. - PubMed
-
- Arend I, Henik A, Okon-Singer H. Dissociating emotion and attention functions in the pulvinar nucleus of the thalamus. Neuropsychology. 2015:29(2):191–196. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

