Proteolytically generated soluble Tweak Receptor Fn14 is a blood biomarker for γ-secretase activity
- PMID: 36069059
- PMCID: PMC9549706
- DOI: 10.15252/emmm.202216084
Proteolytically generated soluble Tweak Receptor Fn14 is a blood biomarker for γ-secretase activity
Abstract
Fn14 is a cell surface receptor with key functions in tissue homeostasis and injury but is also linked to chronic diseases. Despite its physiological and medical importance, the regulation of Fn14 signaling and turnover is only partly understood. Here, we demonstrate that Fn14 is cleaved within its transmembrane domain by the protease γ-secretase, resulting in secretion of the soluble Fn14 ectodomain (sFn14). Inhibition of γ-secretase in tumor cells reduced sFn14 secretion, increased full-length Fn14 at the cell surface, and enhanced TWEAK ligand-stimulated Fn14 signaling through the NFκB pathway, which led to enhanced release of the cytokine tumor necrosis factor. γ-Secretase-dependent sFn14 release was also detected ex vivo in primary tumor cells from glioblastoma patients, in mouse and human plasma and was strongly reduced in blood from human cancer patients dosed with a γ-secretase inhibitor prior to chimeric antigen receptor (CAR)-T-cell treatment. Taken together, our study demonstrates a novel function for γ-secretase in attenuating TWEAK/Fn14 signaling and suggests the use of sFn14 as an easily measurable pharmacodynamic biomarker to monitor γ-secretase activity in vivo.
Keywords: Alzheimer's disease; TNR12; ectodomain shedding; glioblastoma; intramembrane proteolysis.
© 2022 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

Domain structure of Fn14. Fn14 has a short, compact N‐terminal extracellular domain. It consists of 53 amino acids that contain three disulfide bridges (dashed lines) that form the cysteine‐rich domain, CRD (red). Fn14 has a single transmembrane domain (blue) and a short C‐terminal cytosolic tail with a TRAF binding site (green) important for its signaling function. Protein N‐ and C‐termini (N, C) are indicated.
Detection of sFn14 in conditioned media. HEK293E cells were transfected with either empty vector or a plasmid encoding human Fn14 that bears an N‐terminal HA‐tag and a C‐terminal double FLAG‐tag. Conditioned media and lysates of the transfected cells were collected and analyzed by immunoblotting with the indicated antibodies. Calnexin served as a loading control. Shown are representative blots from N = 3 experiments.
Generation of sFn14 is sensitive to the γ‐secretase inhibitor DAPT. HEK293E cells were transfected with either Fn14 or C99 (C‐terminal fragment of APP), both containing a N‐terminal HA‐tag and C‐terminal double FLAG‐tag. One day after transfection, cells were treated with γ‐secretase inhibitor DAPT (1 μM), broad‐spectrum metalloprotease inhibitor TAPI‐1 (50 μM), or the corresponding amount of vehicle DMSO as indicated. The conditioned media and the lysates were blotted with anti‐HA antibody. Shown are representative blots from N = 4 experiments.
Schematic representation of Fn14 shedding by the γ‐secretase complex. γ‐Secretase is a hetero‐tetrameric complex consisting of the indicated subunits, with presenilin being the catalytic subunit and the ectodomain of nicastrin forming a lid‐like structure on top of the γ‐secretase complex. Proteolysis takes place at the catalytic core (indicated by black arrow head), within the lipid bilayer. Proteolysis results in one fragment released into the extracellular space (sFn14), and one fragment into the cytosol (Fn14 ICD, intracellular domain).
In vitro γ‐secretase cleavage assay. HEK293E cells were transfected with epitope‐tagged Fn14 or C99. Cellular membranes were collected and incubated under indicated conditions for the γ‐secretase activity assay. Reactions were terminated and ultracentrifuged. Supernatant (containing γ‐secretase cleavage products) was used for detecting the ICD fragment while the pellet was used to blot for full‐length proteins Fn14 or C99. Blotting is done by anti‐FLAG antibody. Shown are representative blots from N = 3 experiments.
sFn14 production requires the proteolytic presenilin subunit of γ‐secretase. HEK293 cells stably transfected with APP carrying the Swedish double mutation (K595N/M596L) and with a CRISPR/CAS9‐mediated knockout of presenilin 1 (PS1 KO) or of both PS1 and PS2 (PS1/2 dKO), were transiently transfected with epitope‐tagged Fn14. Conditioned media and lysates of the transfected cells were blotted with the indicated antibodies. C83 is a small C‐terminal fragment of APP (containing the C‐terminal 83 amino acids), which is also subjected to γ‐secretase processing. Upon longer exposure, the C99 (generated by BACE1 from APP) is also visible in the PS1/2 dKO condition. Shown are representative blots from N = 4 experiments.
Quantification of results from panel (F). sFn14 levels in the conditioned media quantified and normalized to WT sFn14 signal.

Using an anti‐HA‐epitope antibody, sFn14 was immunoprecipitated from the conditioned medium of HEK293E cells that were untransfected (Control) or transiently transfected with an HA‐tagged Fn14‐expressing construct and additionally treated overnight with DMSO or DAPT (1 μM). The m/z values of each peak are indicated as well as—above them—the amino acid numbers of the peptide fragment corresponding to the amino acid numbers of wild‐type Fn14. The asterisk (*) labels the peaks that show a mass shift that can be explained by phosphorylation of the annotated fragment. Data are averaged from N = 4 experiments.
Detected sFn14 peptides generated by γ‐secretase are listed with their theoretical (theor.) and observed (obser.) mass and the mass difference (Δm) between theoretical and observed mass. The corresponding peptide sequence is also indicated. “*” indicates the peptides with the mass shift that corresponds to addition of a phosphate group to the peptide. The underlined text highlights the transmembrane domain of Fn14 as annotated in UniProt. Three intact disulfide bridges are included in the mass calculations, while methionines were considered nonoxidized in the theoretical mass.
Schematic representation of the γ‐secretase cleavage sites in Fn14. The underlined sequence indicates the transmembrane domain of Fn14, according to Uniprot. Arrows indicate the cleavage sites. The larger arrows show the cleavage sites with the higher intensity after amino acids 48 and 56. Numbers of amino acids refer to full‐length Fn14 after signal peptide cleavage, which removes the first 27 amino acids of the Uniprot‐annotated sequence. The C‐terminal peptide (DF…ILG) which was generated upon AspN cleavage and used for fragmentation is highlighted in bold.
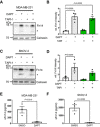
Human breast cancer cell line MDA‐MB‐231 shows cellular accumulation of Fn14 upon γ‐secretase inhibition. The cells were treated overnight with γ‐secretase inhibitor DAPT (1 μM), broad‐spectrum metalloprotease inhibitor TAPI‐1 (50 μM), or the corresponding amount of vehicle DMSO as indicated. Lysates were blotted for Fn14 with an antibody that targets the C‐terminal end of the protein, or against calnexin as loading control. The asterisk labels an N‐terminally truncated form of Fn14.
Quantification of blots from panel (A). The control condition, where the cells were only treated with vehicle (DMSO), was used as baseline, and its average normalized to 1.
Human ovarian cancer cell line SKOV‐3 shows cellular accumulation of Fn14 upon γ‐secretase inhibition. The cells were treated overnight with γ‐secretase inhibitor DAPT (1 μM), broad‐spectrum metalloprotease inhibitor TAPI‐1 (50 μM), or corresponding amount of vehicle DMSO as indicated. Lysates were blotted for Fn14 with an antibody that targets the C‐terminal end of the protein, or against calnexin as loading control. The asterisk labels an N‐terminally truncated form of Fn14.
Quantification of blot from panel (C). The control condition, where the cells were only treated with vehicle (DMSO), was used as baseline, and its average normalized to 1.
sFn14 is reduced upon γ‐secretase inhibition in MDA‐MB‐231 cells. Conditioned media of the treated cells were collected after overnight DAPT (1 μM) or vehicle treatment. sFn14 concentration was measured by human Fn14 ELISA.
sFn14 is reduced upon γ‐secretase inhibition in SKOV‐3 cells. Conditioned media of the treated cells were collected after 48‐h DAPT (1 μM) or vehicle treatment. sFn14 concentration was measured by human Fn14 ELISA.

sFn14, immunoprecipitated from the conditioned medium of HA‐Fn14‐trasfected HEK293E cells, was digested with AspN. The peptide corresponding to the C‐terminal fragment with the sequence “DFCLGCAAAPPAPFRLLWPILG” was fragmented and sequenced. Detected fragment ions are schematically indicated.
Fragmentation spectrum of the peptide “DFCLGCAAAPPAPFRLLWPILG.” Ion names and measured masses are indicated in the figure and are representative for N = 2 experiments.

- A
Mouse glioblastoma cell line GL261 showed cellular accumulation of Fn14 upon γ‐secretase inhibition. Cells were treated overnight with γ‐secretase inhibitor DAPT (1 μM) or vehicle. Lysates of biological replicates (Rep.) were blotted for Fn14 with an antibody that targets the C‐terminal end of the protein, or against calnexin as loading control.
- B
Quantification of blot in panel (A). Intensity values of Fn14 were normalized to the respective Calnexin loading control. The average of the control condition, where the cells were only treated with vehicle (DMSO), was consecutively normalized to 1.
- C
Conditioned media of the GL261 cells from panel (A) were collected, and sFn14 levels were measured by ELISA.
- D
Mouse breast cancer cell line 4T1 showed cellular accumulation of Fn14 upon γ‐secretase inhibition. Cells were treated overnight with γ‐secretase inhibitor DAPT (1 μM) or vehicle. Lysates were blotted for Fn14 with an antibody that targets the C‐terminal end of the protein, or against calnexin as loading control.
- E
Quantification of blot in panel (D). Intensity values of Fn14 were normalized to the respective calnexin loading control. The average of the control condition, where the cells were only treated with vehicle (DMSO), was consecutively normalized to 1.
- F
Conditioned media of the 4T1 cells from panel (D) were collected and sFn14 levels measured by ELISA.
- G
Mouse ovarian cancer cell line ID8 showed cellular accumulation of Fn14 upon γ‐secretase inhibition. Cells were treated overnight with γ‐secretase inhibitor DAPT (1 μM) or vehicle. Lysates were blotted for Fn14 with an antibody that targets the C‐terminal end of the protein, or against calnexin as loading control.
- H
Quantification of blot in panel (G). Intensity values of Fn14 were normalized to the respective calnexin loading control. The average of the control condition, where the cells were only treated with vehicle (DMSO), was consecutively normalized to 1.
- I
Conditioned media of the ID8 cells from panel (G) were collected and sFn14 levels measured by ELISA.
- J, K
Conditioned media of (J) MDA‐MB‐231 or (K) SKOV‐3 cells were collected at indicated time points after DAPT (1 μM) or vehicle treatment and endogenous sFn14 levels were measured by ELISA. Even after 72 h DAPT still completely blocked γ‐secretase, as evidenced by the lack of sFn14 secretion.

MDA‐MB‐231 cells were transfected with an siRNA pool against human Fn14 or nontargeting control (Ctrl) siRNA. A day after transfection, the cells were treated with γ‐secretase inhibitor DAPT (1 μM) or vehicle overnight. The lysate was collected and blotted against Fn14 (C‐terminal antibody) or calnexin as loading control. Shown are representative blots from N = 4 experiments.
Quantification of blot in panel (A). The control condition where the cells were only treated with vehicle DMSO and nontargeting siRNA (DMSO + siCtrl) was used as baseline, and its average normalized to 1. N = 4 experiments.
MDA‐MB‐231 cells were transfected and treated as in panel (A). The treated cells were suspended and labeled with ITEM‐4 antibody that targets an extracellular site of Fn14, or isotype control. Shown are representative histograms from N = 3 experiments.
The mean intensity of the measurement from panel (C). The control condition where the cells were only treated with vehicle DMSO and nontargeting siRNA (DMSO + siCtrl) was used as baseline, and its average normalized to 1. N = 3 experiments.

- A, B
MDA‐MB‐231 cells were transfected with an siRNA pool against human Fn14 or nontargeting control (Ctrl) siRNA. A day after transfection, cells were treated with γ‐secretase inhibitor DAPT (1 μM) or vehicle overnight. TWEAK (100 ng/ml) was applied for indicated time points. Corresponding immunoblots are in Fig EV3 panel (A) and were used for quantification of (A) IκB and (B) pIκB. The measurements were normalized to the 0 min time point (N = 4).
- C
Activation of NFκB is represented as ratio of pIκB to total IκB after TWEAK stimulation (N = 4).
- D
NFκB stimulation mediated by TNF is independent of Fn14 and γ‐secretase. MDA‐MB‐231 cells were treated with TNF (10 ng/ml) for 10 min, and the NFκB activation is reported as ratio of pIκB to total IκB (N = 4).
- E
MDA‐MB‐231 cells were treated with γ‐secretase inhibitor DAPT (1 μM) or vehicle overnight. TWEAK (100 ng/ml) was applied for 10 min. Quantification of the P65 (NFκB) and pP65 blots in Fig EV3 panel (B). The measurements were normalized to the 0 min time point (N = 3).
- F
SKOV‐3 cells were treated with DAPT (1 μM) or vehicle for 48 h and stimulated overnight with TWEAK (100 ng/ml). Conditioned media of the cells were collected, and secreted TNF was measured by ELISA. Shown are data from N = 3 experiments.
- G
SKOV‐3 cells were transfected with Luciferase constructs, switched to low serum, treated with DAPT (1 μM) or vehicle overnight with and stimulated with TWEAK (100 ng/ml) for 4 h. Cells were harvested and NFκB reporter activation was measured using a luminometer (N = 7).

MDA‐MB‐231 cells were transfected with an siRNA pool against human Fn14 or nontargeting control (Ctrl) siRNA. A day after transfection, cells were treated with γ‐secretase inhibitor DAPT (1 μM) or vehicle overnight. Either TWEAK (100 ng/ml) or positive control TNF (10 ng/ml) were applied for indicated time points. The cell lysate was blotted against pIκB and IκB to evaluate NFκB activation or against Fn14 to verify the effect of the DAPT and siFn14 treatment, or against calnexin as a loading control. Shown are representative blots from N = 4 experiments.
MDA‐MB‐231 cells were treated with γ‐secretase inhibitor DAPT (1 μM) or vehicle overnight. TWEAK (100 ng/ml) was applied for 10 min. The cell lysate was blotted against pP65 and P65 to evaluate NFκB activation or against β‐actin as a loading control. Shown are representative blots from N = 3 experiments. The dashed vertical line indicates that sample were run on the same blot but not directly next to each other.

- A
U87 cells were treated with γ‐secretase inhibitor DAPT (1 μM) or vehicle overnight. Either TWEAK (100 ng/ml) or positive control TNF (10 ng/ml) were applied for indicated time points. The cell lysate was blotted against pIκB and IκB to evaluate NFκB activation or against Fn14 to verify the effect of the DAPT and siFn14 treatment, or against calnexin as a loading control. Shown are representative blots from N = 4–5 experiments.
- B
U87 cells showed cellular accumulation of Fn14 upon γ‐secretase inhibition. 0 min time point samples of Fn14 blot in panel (A) were quantified, normalized to the respective calnexin loading control and consecutively normalized to vehicle control average. Shown is the Fn14 intensity relative (rel.) to the DMSO control (N = 4).
- C
γ‐Secretase inhibition by DAPT does not alter NFκB stimulation through TNF. U87 cells treated with TNF (10 ng/ml) for 10 min and the NFκB activation reported as ratio of pIκB to total IκB. Shown is the pIκB/IκB ratio relative (rel.) to the DMSO control (N = 5).
- D, E
Quantification of the IκB (D) and pIκB (E) blots in panel (A). The measurements were normalized to the 0 min time point. N = 5 biological replicates.
- F
The TWEAK stimulation of Fn14 and activation of NFκB is represented as ratio of pIκB to total IκB, taken from quantifications in (D) and (E). Shown is the pIκB/IκB ratio relative (rel.) to the 0 min time point. N = 5 biological replicates.

Cellular Fn14 in ex vivo glioblastoma samples increased upon γ‐secretase inhibition. Primary cells from four different glioblastomas were treated with DAPT (1 μM) or vehicle overnight. Lysates were blotted against Fn14 and calnexin as loading control.
Quantification of the blot in panel (A). For each glioblastoma, the relative (rel.) mean intensity of the normalized Fn14 vehicle condition was used for normalization.
sFn14 was strongly reduced upon γ‐secretase inhibition in primary GBM cells. Conditioned media of the treated cells from panel (A) were collected after overnight DAPT (1 μM) or vehicle treatment. sFn14 levels in these samples were measured by human Fn14 ELISA.
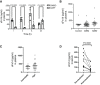
Mice were treated with single dose of 100 mg/kg DAPT (or vehicle DMSO) and their plasma was collected at the indicated time points after dosing. Individual mice were used at each time point. sFn14 levels in these samples are displayed (N = 4).
Plasma samples from 10 controls and GBM patients with an initial diagnosis of GBM (iGBM, 40 patients) or a recurrent GBM (rGBM, 20 patients) were collected and sFn14 levels were measured by ELISA.
Plasma samples from 10 ovarian cancer patients with low/middle and 19 patients with high tumor burden were collected and sFn14 levels were measured by ELISA. The tumor burden was clinically determined by computer tomographic scans.
Serum samples from 10 patients with refractory multiple myeloma were collected pre‐ and posttreatment with 25 mg LY3039478 administered in three daily doses over 5 days. sFn14 levels in these samples are displayed (N = 10).

Under normal conditions, Fn14 is processed by γ‐secretase, which generates sFn14 and degrades Fn14. Fn14 ligand TWEAK binds to remaining Fn14 on the surface, which activates downstream NFκB signaling.
Upon inhibition of γ‐secretase activity, Fn14 proteolysis is reduced. This ablates sFn14 release and increases Fn14 in the cells and on the cell surface, allowing enhanced Fn14‐mediated signaling upon TWEAK ligand binding.
References
-
- Aggarwal BB (2003) Signalling pathways of the TNF superfamily: a double‐edged sword. Nat Rev Immunol 3: 745–756 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

