Effect of LHRH analogs on lower urinary tract symptoms associated with advanced prostate cancer in real clinical practice: ANALUTS study
- PMID: 36069170
- PMCID: PMC9826298
- DOI: 10.1002/nau.25031
Effect of LHRH analogs on lower urinary tract symptoms associated with advanced prostate cancer in real clinical practice: ANALUTS study
Abstract
Aims: To estimate the prevalence of lower urinary tract symptoms (LUTS) in patients with prostate cancer scheduled to receive LHRH analogs, and to assess the effectiveness of LHRH analogs on LUTS in patients presenting moderate/severe symptoms.
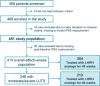
Methods: Prospective, noninterventional, multicenter study conducted at 28 centers in Spain and Portugal. LUTS were evaluated using the International Prostate Symptom Score (IPSS) at baseline, 24 and 48 weeks after initiation of treatment. Subanalyses were performed according to age and concomitant treatment (radiotherapy, alpha-blockers, and antiandrogens).
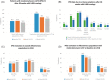
Results: A total of 354 patients were treated with LHRH analogs for 48 weeks. The percentage of patients with moderate/severe LUTS (IPSS > 7) decreased from 60.2% (n = 213/354) at baseline to 52.8% (n = 187/354) at Week 48. Among patients with moderate/severe LUTS at baseline: 73.7% (n = 157/213) still had moderate/severe LUTS at Week 48; percentage reductions of patients with LUTS at Week 48 were statistically significant (p < 0.05) overall and by age or concomitant treatment, except for alpha-blockers (84.2% patients receiving them still had moderate/severe LUTS at Week 48). All IPSS items, including quality of life for urinary symptoms, improved throughout the study. The only predictor of response to treatment with LHRH analogs that improved IPSS by 3 points after 48 weeks was baseline testosterone levels. Lower baseline testosterone levels were associated with greater improvement in IPSS after treatment with LHRH analogs (odds ratio 0.998, 95% confidence interval 0.996-1.000, p = 0.0277).
Conclusion: LHRH analogs have a positive effect in patients with locally advanced or metastatic prostate cancer presenting moderate/severe LUTS regardless of age or concomitant treatment received (radiotherapy, antiandrogens, or alpha-blockers).
Keywords: androgen deprivation; concomitant; hormonal therapy; quality of life; radiotherapy.
© 2022 The Authors. Neurourology and Urodynamics published by Wiley Periodicals LLC.
Conflict of interest statement
Juan Morote, Antonio Gómez‐Caamaño, Raúl Poza de Celis, Francisco Gómez‐Veiga, Juan Pablo Ciria, Jesús Calleja, Javier Extramiana and Javier C. Angulo have no disclosure. Maria Pérez‐Sampietro and Valerie Perrot are employees of Ipsen Pharma.
Figures
References
-
- Walz J, Suardi N, Hutterer GC, et al. Lower urinary tract symptoms affect one‐third of men in a prostate cancer screening population. J Endourol. 2008;22(2):369‐376. - PubMed
-
- Fransson P. Patient‐reported lower urinary tract symptoms, urinary incontinence, and quality of life after external beam radiotherapy for localized prostate cancer ‐ 15 years’ follow‐up. A comparison with age‐matched controls. Acta Oncol. 2008;47(5):852‐861. - PubMed
-
- Crawford ED, Kavanagh BD. The role of alpha‐blockers in the management of lower urinary tract symptoms in prostate cancer patients treated with radiation therapy. Am J Clin Oncol. 2006;29(5):517‐523. - PubMed
-
- Blaivas JG, Weiss JP, Jones M. The pathophysiology of lower urinary tract symptoms after brachytherapy for prostate cancer. BJU Int. 2006;98(6):1233‐1237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

