Off-Label, but on-Evidence? A Review of the Level of Evidence for Pediatric Pharmacotherapy
- PMID: 36069288
- PMCID: PMC9828396
- DOI: 10.1002/cpt.2736
Off-Label, but on-Evidence? A Review of the Level of Evidence for Pediatric Pharmacotherapy
Abstract
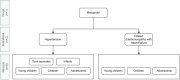
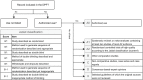
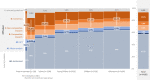
Many drugs are still prescribed off-label to the pediatric population. Although off-label drug use not supported by high level of evidence is potentially harmful, a comprehensive overview of the quality of the evidence pertaining off-label drug use in children is lacking. The Dutch Pediatric Formulary (DPF) provides best evidence-based dosing guidelines for drugs used in children. For each drug-indication-age group combination-together compiling one record-we scored the highest available level of evidence: labeled use, systematic review or meta-analysis, randomized controlled trial (RCT), comparative research, noncomparative research, or consensus-based expert opinions. For records based on selected guidelines, the original sources were not reviewed. These records were scored as guideline. A total of 774 drugs were analyzed comprising a total of 6,426 records. Of all off-label records (n = 2,718), 14% were supported by high quality evidence (4% meta-analysis or systematic reviews, 10% RCTs of high quality), 20% by comparative research, 14% by noncomparative research, 37% by consensus-based expert opinions, and 15% by selected guidelines. Fifty-eight percent of all records were authorized, increasing with age from 30% in preterm neonates (n = 110) up to 64% in adolescents (n = 1,630). Many have advocated that off-label use is only justified when supported by a high level of evidence. We show that this prerequisite would seriously limit available drug treatment for children as the underlying evidence is low across ages and drug classes. Our data identify the drugs and therapeutic areas for which evidence is clearly missing and could drive the global research agenda.
© 2022 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
T.vdZ. is managing director of the Dutch Knowledge Center Pharmacotherapy for Children. S.dW. is medical director of Dutch Knowledge Center Pharmacotherapy for Children All other authors declared no competing interests for this work.
Figures

References
-
- Regulation (EC) no 1901/2006 of the European Parliament and of the Council on Medicinal Products for Pediatric Use and amending Regulation (EEC) no 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) no 726/2004, EC/190 (2006).
-
- Carmack, M. , Hwang, T. & Bourgeois, F.T. Pediatric drug policies supporting safe and effective use of therapeutics in children: a systematic analysis. Health Aff (Millwood) 39, 1799–1805 (2020). - PubMed
-
- Frattarelli, D.A. et al. Off‐label use of drugs in children. Pediatrics 133, 563–567 (2014). - PubMed
-
- Weda, M . et al. Study on the off‐label use of medicinal products in the European Union <www.ec.europa.com> Updated March 14, 2017.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

