Tumor-associated macrophages in liver cancer: From mechanisms to therapy
- PMID: 36069342
- PMCID: PMC9648394
- DOI: 10.1002/cac2.12345
Tumor-associated macrophages in liver cancer: From mechanisms to therapy
Abstract
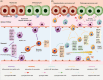
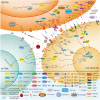
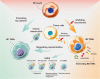
Multidimensional analyses have demonstrated the presence of a unique tumor microenvironment (TME) in liver cancer. Tumor-associated macrophages (TAMs) are among the most abundant immune cells infiltrating the TME and are present at all stages of liver cancer progression, and targeting TAMs has become one of the most favored immunotherapy strategies. In addition, macrophages and liver cancer cells have distinct origins. At the early stage of liver cancer, macrophages can provide a niche for the maintenance of liver cancer stem cells. In contrast, cancer stem cells (CSCs) or poorly differentiated tumor cells are key factors modulating macrophage activation. In the present review, we first propose the origin connection between precursor macrophages and liver cancer cells. Macrophages undergo dynamic phenotypic transition during carcinogenesis. In this course of such transition, it is critical to determine the appropriate timing for therapy and block specific markers to suppress pro-tumoral TAMs. The present review provides a more detailed discussion of transition trends of such surface markers than previous reviews. Complex crosstalk occurs between TAMs and liver cancer cells. TAMs play indispensable roles in tumor progression, angiogenesis, and autophagy due to their heterogeneity and robust plasticity. In addition, macrophages in the TME interact with other immune cells by directing cell-to-cell contact or secreting various effector molecules. Similarly, tumor cells combined with other immune cells can drive macrophage recruitment and polarization. Despite the latest achievements and the advancements in treatment strategies following TAMs studies, comprehensive discussions on the communication between macrophages and cancer cells or immune cells in liver cancer are currently lacking. In this review, we discussed the interactions between TAMs and liver cancer cells (from cell origin to maturation), the latest therapeutic strategies (including chimeric antigen receptor macrophages), and critical clinical trials for hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA) to provide a rationale for further clinical investigation of TAMs as a potential target for treating patients with liver cancer.
Keywords: hepatocellular carcinoma; immunotherapy; intrahepatic cholangiocarcinoma; tumor-associated macrophages.
© 2022 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
Figures



References
-
- Seton‐Rogers S. Taming TAMs in brain metastases. Nat Rev Cancer. 2022;22(1):2–3. - PubMed
-
- Sunakawa Y, Stintzing S, Cao S, Heinemann V, Cremolini C, Falcone A, et al. Variations in genes regulating tumor‐associated macrophages (TAMs) to predict outcomes of bevacizumab‐based treatment in patients with metastatic colorectal cancer: results from TRIBE and FIRE3 trials. Ann Oncol. 2015;26(12):2450–6. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

