Sexual Dimorphism in the Extracellular and Pericellular Matrix of Articular Cartilage
- PMID: 36069595
- PMCID: PMC9459468
- DOI: 10.1177/19476035221121792
Sexual Dimorphism in the Extracellular and Pericellular Matrix of Articular Cartilage
Abstract
Objective: Women have a higher prevalence and burden of joint injuries and pathologies involving articular cartilage than men. Although knee injuries affecting young women are on the rise, most studies related to sexual dimorphism target postmenopausal women. We hypothesize that sexual dimorphism in cartilage structure and mechanics is present before menopause, which can contribute to sex disparities in cartilage pathologies.
Design: Bovine knee was used as a model to study healthy adult cartilage. We compared elastic moduli under compression, abundances of extracellular and pericellular matrix (PCM) proteins using proteomics, and PCM constituency with tissue immunofluorescence. The gene expression of matrix-related genes under basal, anabolic, and catabolic conditions was assessed by quantitative polymerase chain reaction (qPCR).
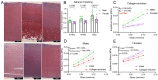
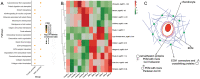
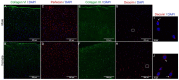
Results: The equilibrium modulus was higher in male cartilage compared with female cartilage. Proteoglycans were not associated with this biomechanical dimorphism. Proteomic and pathway analyses of tissue showed dimorphic enriched pathways in extracellular matrix (ECM)-related proteins in which male cartilage was enriched in matrix interconnectors and crosslinkers that strengthen the ECM network. Moreover, male and female tissue differed in enriched PCM components. Females had more abundance of collagen type VI and decorin, suggesting different PCM mechanics. Furthermore, the activation of regenerative and catabolic function in chondrocytes triggered sex-dependent signatures in gene expression, indicating dimorphic genetic regulation that is dependent on stimulation.
Conclusions: We provide evidence for sexual dimorphism in cartilage before menopause. Some differences are intrinsic to chondrocytes' gene expression defined by their XX versus XY chromosomal constituency.
Keywords: articular cartilage; extracellular matrix; pericellular matrix; sex differences.
Conflict of interest statement
Figures





References
-
- Racine J, Aaron RK. Post-traumatic osteoarthritis after ACL injury. R I Med J. 2014;97(11):25-8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

