A randomized trial of blood donor iron repletion on red cell quality for transfusion and donor cognition and well-being
- PMID: 36069596
- PMCID: PMC9837440
- DOI: 10.1182/blood.2022017288
A randomized trial of blood donor iron repletion on red cell quality for transfusion and donor cognition and well-being
Abstract
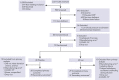
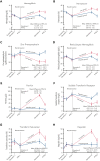
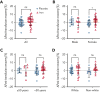
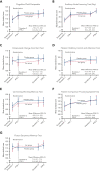
Although altruistic regular blood donors are vital for the blood supply, many become iron deficient from donation-induced iron loss. The effects of blood donation-induced iron deficiency on red cell transfusion quality or donor cognition are unknown. In this double-blind, randomized trial, adult iron-deficient blood donors (n = 79; ferritin < 15 μg/L and zinc protoporphyrin >60 μMol/mol heme) who met donation qualifications were enrolled. A first standard blood donation was followed by the gold-standard measure for red cell storage quality: a 51-chromium posttransfusion red cell recovery study. Donors were then randomized to intravenous iron repletion (1 g low-molecular-weight iron dextran) or placebo. A second donation ∼5 months later was followed by another recovery study. Primary outcome was the within-subject change in posttransfusion recovery. The primary outcome measure of an ancillary study reported here was the National Institutes of Health Toolbox-derived uncorrected standard Cognition Fluid Composite Score. Overall, 983 donors were screened; 110 were iron-deficient, and of these, 39 were randomized to iron repletion and 40 to placebo. Red cell storage quality was unchanged by iron repletion: mean change in posttransfusion recovery was 1.6% (95% confidence interval -0.5 to 3.8) and -0.4% (-2.0 to 1.2) with and without iron, respectively. Iron repletion did not affect any cognition or well-being measures. These data provide evidence that current criteria for blood donation preserve red cell transfusion quality for the recipient and protect adult donors from measurable effects of blood donation-induced iron deficiency on cognition. This trial was registered at www.clinicaltrials.gov as NCT02889133 and NCT02990559.
© 2022 by The American Society of Hematology.
Conflict of interest statement
Conflicts of interest disclosure: The authors declare no competing financial interests.
Figures

Comment in
-
To Fe, or not to Fe, that is the question.Blood. 2022 Dec 22;140(25):2658-2660. doi: 10.1182/blood.2022017881. Blood. 2022. PMID: 36548014 Free PMC article. No abstract available.
References
-
- Szczepiorkowski Z, Hopkins T. Updated strategies to limit or prevent iron deficiency in blood donors, Association bulletin #17-02. https://www.aabb.org/docs/default-source/default-document-library/resour...
-
- Moroff G, Sohmer PR, Button LN. Proposed standardization of methods for determining the 24-hour survival of stored red cells. Transfusion. 1984;24(2):109–114. - PubMed
-
- Hess JR, Biomedical Excellence for Safer Transfusion C Scientific problems in the regulation of red blood cell products. Transfusion. 2012;52(8):1827–1835. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

