A Robust, Highly Multiplexed Mass Spectrometry Assay to Identify SARS-CoV-2 Variants
- PMID: 36069609
- PMCID: PMC9604185
- DOI: 10.1128/spectrum.01736-22
A Robust, Highly Multiplexed Mass Spectrometry Assay to Identify SARS-CoV-2 Variants
Abstract
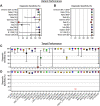
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants are characterized by differences in transmissibility and response to therapeutics. Therefore, discriminating among them is vital for surveillance, infection prevention, and patient care. While whole-genome sequencing (WGS) is the "gold standard" for variant identification, molecular variant panels have become increasingly available. Most, however, are based on limited targets and have not undergone comprehensive evaluation. We assessed the diagnostic performance of the highly multiplexed Agena MassARRAY SARS-CoV-2 Variant Panel v3 to identify variants in a diverse set of 391 SARS-CoV-2 clinical RNA specimens collected across our health systems in New York City, USA and Bogotá, Colombia (September 2, 2020 to March 2, 2022). We demonstrated almost perfect levels of interrater agreement between this assay and WGS for 9 of 11 variant calls (κ ≥ 0.856) and 25 of 30 targets (κ ≥ 0.820) tested on the panel. The assay had a high diagnostic sensitivity (≥93.67%) for contemporary variants (e.g., Iota, Alpha, Delta, and Omicron [BA.1 sublineage]) and a high diagnostic specificity for all 11 variants (≥96.15%) and all 30 targets (≥94.34%) tested. Moreover, we highlighted distinct target patterns that could be utilized to identify variants not yet defined on the panel, including the Omicron BA.2 and other sublineages. These findings exemplified the power of highly multiplexed diagnostic panels to accurately call variants and the potential for target result signatures to elucidate new ones. IMPORTANCE The continued circulation of SARS-CoV-2 amid limited surveillance efforts and inconsistent vaccination of populations has resulted in the emergence of variants that uniquely impact public health systems. Thus, in conjunction with functional and clinical studies, continuous detection and identification are quintessential to informing diagnostic and public health measures. Furthermore, until WGS becomes more accessible in the clinical microbiology laboratory, the ideal assay for identifying variants must be robust, provide high resolution, and be adaptable to the evolving nature of viruses like SARS-CoV-2. Here, we highlighted the diagnostic capabilities of a highly multiplexed commercial assay to identify diverse SARS-CoV-2 lineages that circulated from September 2, 2020 to March 2, 2022 among patients seeking care in our health systems. This assay demonstrated variant-specific signatures of nucleotide/amino acid polymorphisms and underscored its utility for the detection of contemporary and emerging SARS-CoV-2 variants of concern.
Keywords: MALDI-TOF; Omicron; RT-PCR; SARS-CoV-2; multiplex; variant panel.
Conflict of interest statement
The authors declare a conflict of interest. Robert Sebra is VP of Technology Development and a stockholder at Sema4, a Mount Sinai Venture. This work, however, was conducted solely at Icahn School of Medicine at Mount Sinai. Otherwise, the authors declare no competing interests.
Figures


Update of
-
A robust, highly multiplexed mass spectrometry assay to identify SARS-CoV-2 variants.medRxiv [Preprint]. 2022 May 29:2022.05.28.22275691. doi: 10.1101/2022.05.28.22275691. medRxiv. 2022. Update in: Microbiol Spectr. 2022 Oct 26;10(5):e0173622. doi: 10.1128/spectrum.01736-22. PMID: 35665019 Free PMC article. Updated. Preprint.
References
-
- Deng X, Gu W, Federman S, du Plessis L, Pybus OG, Faria N, Wang C, Yu G, Bushnell B, Pan C-Y, Guevara H, Sotomayor-Gonzalez A, Zorn K, Gopez A, Servellita V, Hsu E, Miller S, Bedford T, Greninger AL, Roychoudhury P, Starita LM, Famulare M, Chu HY, Shendure J, Jerome KR, Anderson C, Gangavarapu K, Zeller M, Spencer E, Andersen KG, MacCannell D, Paden CR, Li Y, Zhang J, Tong S, Armstrong G, Morrow S, Willis M, Matyas BT, Mase S, Kasirye O, Park M, Masinde G, Chan C, Yu AT, Chai SJ, Villarino E, Bonin B, Wadford DA, Chiu CY. 2020. Genomic surveillance reveals multiple introductions of SARS-CoV-2 into Northern California. Science 369:582–587. doi: 10.1126/science.abb9263. - DOI - PMC - PubMed
-
- Hernandez MM, Gonzalez-Reiche AS, Alshammary H, Fabre S, Khan Z, van De Guchte A, Obla A, Ellis E, Sullivan MJ, Tan J, Alburquerque B, Soto J, Wang C-Y, Sridhar SH, Wang Y-C, Smith M, Sebra R, Paniz-Mondolfi AE, Gitman MR, Nowak MD, Cordon-Cardo C, Luksza M, Krammer F, van Bakel H, Simon V, Sordillo EM. 2021. Molecular evidence of SARS-CoV-2 in New York before the first pandemic wave. Nat Commun 12:3463. doi: 10.1038/s41467-021-23688-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

