2-D Molecular Alloy Ru-M (M = Cu, Ag, and Au) Carbonyl Clusters: Synthesis, Molecular Structure, Catalysis, and Computational Studies
- PMID: 36069711
- PMCID: PMC9490753
- DOI: 10.1021/acs.inorgchem.2c02099
2-D Molecular Alloy Ru-M (M = Cu, Ag, and Au) Carbonyl Clusters: Synthesis, Molecular Structure, Catalysis, and Computational Studies
Abstract
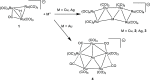
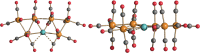
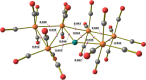
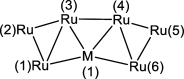
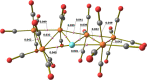
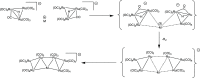
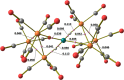
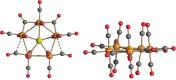
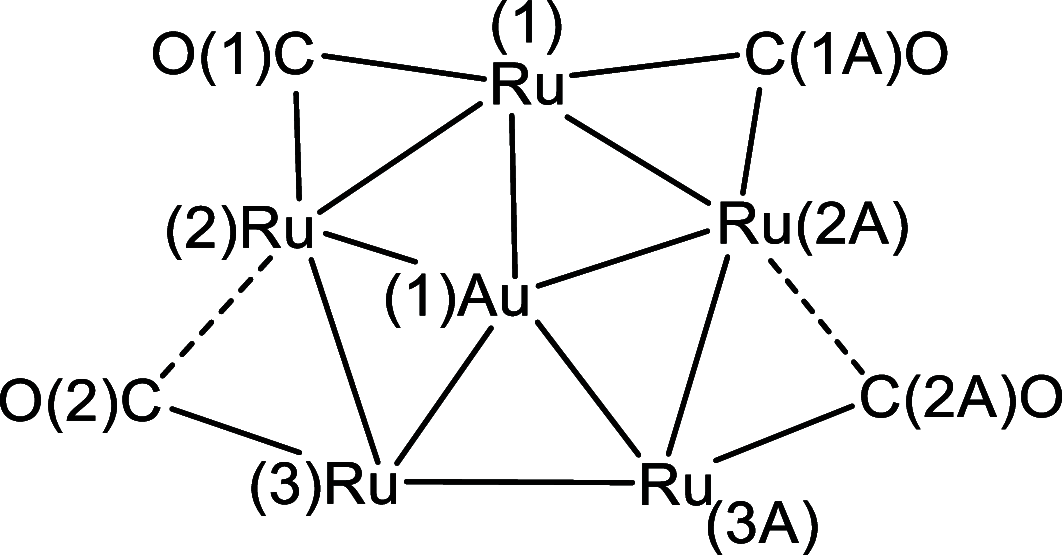
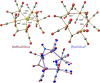
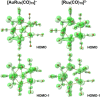
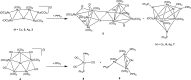
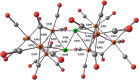
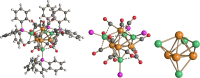
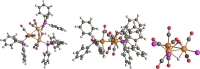
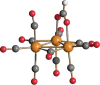
The reactions of [HRu3(CO)11]- (1) with M(I) (M = Cu, Ag, and Au) compounds such as [Cu(CH3CN)4][BF4], AgNO3, and Au(Et2S)Cl afford the 2-D molecular alloy clusters [CuRu6(CO)22]- (2), [AgRu6(CO)22]- (3), and [AuRu5(CO)19]- (4), respectively. The reactions of 2-4 with PPh3 result in mixtures of products, among which [Cu2Ru8(CO)26]2- (5), Ru4(CO)12(CuPPh3)4 (6), Ru4(CO)12(AgPPh3)4 (7), Ru(CO)3(PPh3)2 (8), and HRu3(OH)(CO)7(PPh3)3 (9) have been isolated and characterized. The molecular structures of 2-6 and 9 have been determined by single-crystal X-ray diffraction. The metal-metal bonding within 2-5 has been computationally investigated by density functional theory methods. In addition, the [NEt4]+ salts of 2-4 have been tested as catalyst precursors for transfer hydrogenation on the model substrate 4-fluoroacetophenone using iPrOH as a solvent and a hydrogen source.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
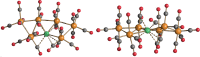
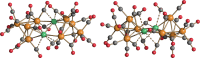
References
-
- Zacchini S. Using Metal Carbonyl Clusters To Develop a Molecular Approach towards Metal Nanoparticles. Eur. J. Inorg. Chem. 2011, 2011, 4125–4145. 10.1002/ejic.201100462. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

