Highly efficient generation of isogenic pluripotent stem cell models using prime editing
- PMID: 36069759
- PMCID: PMC9584603
- DOI: 10.7554/eLife.79208
Highly efficient generation of isogenic pluripotent stem cell models using prime editing
Abstract
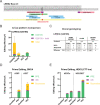
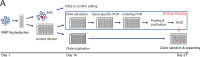
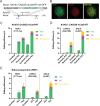
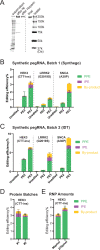
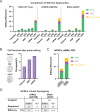
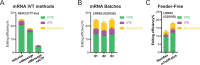
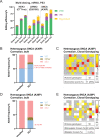
The recent development of prime editing (PE) genome engineering technologies has the potential to significantly simplify the generation of human pluripotent stem cell (hPSC)-based disease models. PE is a multicomponent editing system that uses a Cas9-nickase fused to a reverse transcriptase (nCas9-RT) and an extended PE guide RNA (pegRNA). Once reverse transcribed, the pegRNA extension functions as a repair template to introduce precise designer mutations at the target site. Here, we systematically compared the editing efficiencies of PE to conventional gene editing methods in hPSCs. This analysis revealed that PE is overall more efficient and precise than homology-directed repair of site-specific nuclease-induced double-strand breaks. Specifically, PE is more effective in generating heterozygous editing events to create autosomal dominant disease-associated mutations. By stably integrating the nCas9-RT into hPSCs we achieved editing efficiencies equal to those reported for cancer cells, suggesting that the expression of the PE components, rather than cell-intrinsic features, limit PE in hPSCs. To improve the efficiency of PE in hPSCs, we optimized the delivery modalities for the PE components. Delivery of the nCas9-RT as mRNA combined with synthetically generated, chemically-modified pegRNAs and nicking guide RNAs improved editing efficiencies up to 13-fold compared with transfecting the PE components as plasmids or ribonucleoprotein particles. Finally, we demonstrated that this mRNA-based delivery approach can be used repeatedly to yield editing efficiencies exceeding 60% and to correct or introduce familial mutations causing Parkinson's disease in hPSCs.
Keywords: disease models; genetics; genome engineering; genomics; hPSCs; human; parkinson's disease; prime editing; regenerative medicine; stem cells.
Plain language summary
From muscles to nerves, our body is formed of many kinds of cells which can each respond slightly differently to the same harmful genetic changes. Understanding the exact relationship between mutations and cell-type specific function is essential to better grasp how conditions such as Parkinson’s disease or amyotrophic lateral sclerosis progress and can be treated. Stem cells could be an important tool in that effort, as they can be directed to mature into many cell types in the laboratory. Yet it remains difficult to precisely introduce disease-relevant mutations in these cells. To remove this obstacle, Li et al. focused on prime editing, a cutting-edge ‘search and replace’ approach which can introduce new genetic information into a specific DNA sequence. However, it was unclear whether this technique could be used to efficiently create stem cell models of human diseases. A first set of experiments showed that prime editing is superior to conventional approaches when generating mutated genes in stem cells. Li et al. then further improved the efficiency and precision of the method by tweaking how prime editing components are delivered into the cells. The refined approach could be harnessed to quickly generate large numbers of stem cells carrying mutations associated with Parkinson’s disease; crucially, prime editing could then also be used to revert a mutated gene back to its healthy form. The improved prime editing approach developed by Li et al. removes a major hurdle for scientists hoping to use stem cells to study genetic diseases. This could potentially help to unlock progress in how we understand and ultimately treat these conditions.
© 2022, Li, Busquets et al.
Conflict of interest statement
HL, OB, YV, KS, NK, GP, LG, HB, DR, DH, FS No competing interests declared
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

