Retinoic acid receptor structures: the journey from single domains to full-length complex
- PMID: 36069789
- PMCID: PMC11376212
- DOI: 10.1530/JME-22-0113
Retinoic acid receptor structures: the journey from single domains to full-length complex
Abstract
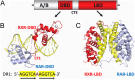
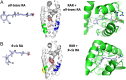
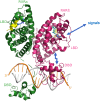
The retinoic acid receptors (RARα, β, and γ) are multi-domain polypeptides that heterodimerize with retinoid X receptors (RXRα, β, and γ) to form functional transcription factors. Understanding the three-dimensional molecular organization of these nuclear receptors (NRs) began with RAR and RXR DNA-binding domains (DBDs), and were followed with studies on isolated ligand-binding domains (LBDs). The more complete picture emerged in 2017 with the multi-domain crystal structure of RXRα-RARβ on its response element with retinoic acid molecules and coactivator segments on both proteins. The analysis of that structure and its complementary studies have clarified the direct communication pathways within RXR-RAR polypeptides, through which DNA binding, protein-ligand, and protein-protein interactions are integrated for overall functional responses. Understanding the molecular connections in the RXR-RAR complex has benefited from direct observations of the multi-domain structures of RXRα-PPARγ, RXRα-LXRβ, HNF-4α homodimer, and androgen receptor homodimer, each bound to its response element. These comprehensive NR structures show unique quaternary architectures, yet all have DBD-DBD, LBD-LBD, and DBD-LBD domain-domain contacts within them. These convergence zones allow signals from discrete domains of their polypeptides to be propagated and integrated across their entire complex, shaping their overall responses in an allosteric fashion.
Keywords: hormone receptors; receptors; retinoic acid; structure.
Conflict of interest statement
The author is a founder and consultant for Flare Therapeutics.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

