Structure of full-length TSH receptor in complex with antibody K1-70™
- PMID: 36069797
- PMCID: PMC9782461
- DOI: 10.1530/JME-22-0120
Structure of full-length TSH receptor in complex with antibody K1-70™
Abstract
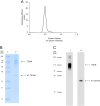
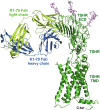
Determination of the full-length thyroid-stimulating hormone receptor (TSHR) structure by cryo-electron microscopy (cryo-EM) is described. The TSHR complexed with human monoclonal TSHR autoantibody K1-70™ (a powerful inhibitor of TSH action) was detergent solubilised, purified to homogeneity and analysed by cryo-EM. The structure (global resolution 3.3 Å) is a monomer with all three domains visible: leucine-rich domain (LRD), hinge region (HR) and transmembrane domain (TMD). The TSHR extracellular domain (ECD, composed of the LRD and HR) is positioned on top of the TMD extracellular surface. Extensive interactions between the TMD and ECD are observed in the structure, and their analysis provides an explanation of the effects of various TSHR mutations on TSHR constitutive activity and on ligand-induced activation. K1-70™ is seen to be well clear of the lipid bilayer. However, superimposition of M22™ (a human monoclonal TSHR autoantibody which is a powerful stimulator of the TSHR) on the cryo-EM structure shows that it would clash with the bilayer unless the TSHR HR rotates upwards as part of the M22™ binding process. This rotation could have an important role in TSHR stimulation by M22™ and as such provides an explanation as to why K1-70™ blocks the binding of TSH and M22™ without activating the receptor itself.
Keywords: TSHR; autoantibodies; autoimmunity; cryo-EM; structure.
Figures


References
-
- Ballesteros JA, Weinstein H.1995Integrated methods for the construction of three dimensional models and computational probing of structure function relations in G protein-coupled receptors. Methods in Neurosciences 25366–428. (10.1016/S1043-9471(0580049-7) - DOI
-
- BIOVIA Dassault Systèmes 2021Discovery Studio 2021. San Diego, CA, USA: BIOVIA Dassault Systèmes.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

