GPX4 regulates cellular necrosis and host resistance in Mycobacterium tuberculosis infection
- PMID: 36069923
- PMCID: PMC9458471
- DOI: 10.1084/jem.20220504
GPX4 regulates cellular necrosis and host resistance in Mycobacterium tuberculosis infection
Abstract
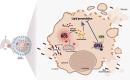
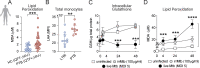
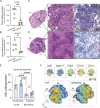
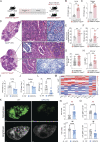
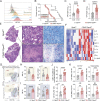
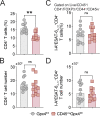
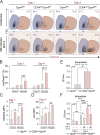
Cellular necrosis during Mycobacterium tuberculosis (Mtb) infection promotes both immunopathology and bacterial dissemination. Glutathione peroxidase-4 (Gpx4) is an enzyme that plays a critical role in preventing iron-dependent lipid peroxidation-mediated cell death (ferroptosis), a process previously implicated in the necrotic pathology seen in Mtb-infected mice. Here, we document altered GPX4 expression, glutathione levels, and lipid peroxidation in patients with active tuberculosis and assess the role of this pathway in mice genetically deficient in or overexpressing Gpx4. We found that Gpx4-deficient mice infected with Mtb display substantially increased lung necrosis and bacterial burdens, while transgenic mice overexpressing the enzyme show decreased bacterial loads and necrosis. Moreover, Gpx4-deficient macrophages exhibited enhanced necrosis upon Mtb infection in vitro, an outcome suppressed by the lipid peroxidation inhibitor, ferrostatin-1. These findings provide support for the role of ferroptosis in Mtb-induced necrosis and implicate the Gpx4/GSH axis as a target for host-directed therapy of tuberculosis.
© 2022 Amaral et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures





References
-
- Amaral, E.P., Conceicao E.L., Costa D.L., Rocha M.S., Marinho J.M., Cordeiro-Santos M., D’Imperio-Lima M.R., Barbosa T., Sher A., and Andrade B.B.. 2016. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol. 16:251. 10.1186/s12866-016-0872-7 - DOI - PMC - PubMed
-
- Amaral, E.P., Costa D.L., Namasivayam S., Riteau N., Kamenyeva O., Mittereder L., Mayer-Barber K.D., Andrade B.B., and Sher A.. 2019. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J. Exp. Med. 216:556–570. 10.1084/jem.20181776 - DOI - PMC - PubMed
-
- Andrade, B.B., Pavan Kumar N., Mayer-Barber K.D., Barber D.L., Sridhar R., Rekha V.V.B., Jawahar M.S., Nutman T.B., Sher A., and Babu S.. 2013. Plasma heme oxygenase-1 levels distinguish latent or successfully treated human tuberculosis from active disease. PLoS One. 8:e62618. 10.1371/journal.pone.0062618 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

