Efficacy and safety of tolvaptan versus placebo in the treatment of patients with autosomal dominant polycystic kidney disease: a meta-analysis
- PMID: 36069961
- PMCID: PMC9958178
- DOI: 10.1007/s11255-022-03353-8
Efficacy and safety of tolvaptan versus placebo in the treatment of patients with autosomal dominant polycystic kidney disease: a meta-analysis
Abstract
Objective: The objective of this meta-analysis was to compare the efficacy and drug safety of tolvaptan with placebo for autosomal dominant polycystic kidney disease (ADPKD).
Methods: The PubMed, Embase, and Cochrane Library databases were searched from inception to September 10, 2021. Eligible studies comparing tolvaptan and placebo in the treatment of patients with ADPKD were included. Data were analysed using Review Manager Version 5.3.
Results: Thirteen studies involving 3575 patients were included in the meta-analysis. Compared with placebo, tolvaptan had a better effect on delaying eGFR decline (MD 1.27, 95% CI 1.24-1.29, P < 0.01) and TKV increase (MD - 3.01, 95% CI - 3.55 to - 2.47, P < 0.01) in ADPKD treatment. Additionally, tolvaptan reduced the incidence of complications such as renal pain (OR 0.71, 95% CI 0.58-0.87, P < 0.01), urinary tract infection (OR 0.69, 95% CI 0.54-0.89, P < 0.01), haematuria (OR 0.68, 95% CI 0.51-0.89, P < 0.01), and hypertension (OR 0.66, 95% CI 0.52-0.82, P < 0.01). However, tolvaptan was associated with a higher incidence rate of adverse events such as thirst (OR 8.48 95% CI 4.53-15.87, P < 0.01), polyuria (OR 4.71, 95% CI 2.17-10.24, P < 0.01), and hepatic injury (OR 4.56, 95% CI 2.51-8.29, P < 0.01).
Conclusion: Tolvaptan can delay eGFR decline and TKV increase and reduce complications such as renal pain, urinary tract infection, haematuria, and hypertension in the treatment of ADPKD. However, tolvaptan increases the adverse effects of thirst, polyuria and hepatic injury.
Keywords: Autosomal dominant polycystic kidney disease; Meta-analysis; Placebo; Tolvaptan.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




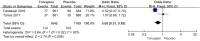


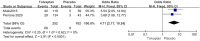
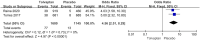
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

