Monocytes maintain central nervous system homeostasis following helminth-induced inflammation
- PMID: 36070344
- PMCID: PMC9478671
- DOI: 10.1073/pnas.2201645119
Monocytes maintain central nervous system homeostasis following helminth-induced inflammation
Abstract
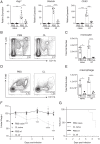
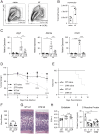
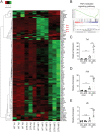
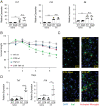
Neuroimmune interactions are crucial for regulating immunity and inflammation. Recent studies have revealed that the central nervous system (CNS) senses peripheral inflammation and responds by releasing molecules that limit immune cell activation, thereby promoting tolerance and tissue integrity. However, the extent to which this is a bidirectional process, and whether peripheral immune cells also promote tolerance mechanisms in the CNS remains poorly defined. Here we report that helminth-induced type 2 inflammation promotes monocyte responses in the brain that are required to inhibit excessive microglial activation and host death. Mechanistically, infection-induced monocytes express YM1 that is sufficient to inhibit tumor necrosis factor production from activated microglia. Importantly, neuroprotective monocytes persist in the brain, and infected mice are protected from subsequent lipopolysaccharide-induced neuroinflammation months after infection-induced inflammation has resolved. These studies demonstrate that infiltrating monocytes promote CNS homeostasis in response to inflammation in the periphery and demonstrate that a peripheral infection can alter the immunologic landscape of the host brain.
Keywords: helminth; innate immune cells; monocyte; neuroimmune cross-talk.
Conflict of interest statement
Competing interest statement: M.C.S. is the President of NemaGen Discoveries.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

