An Evaluation of Sexual Function in the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia in Men Treated with the Temporarily Implanted Nitinol Device
- PMID: 36070450
- PMCID: PMC9810348
- DOI: 10.1089/end.2022.0226
An Evaluation of Sexual Function in the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia in Men Treated with the Temporarily Implanted Nitinol Device
Abstract
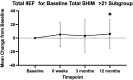
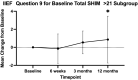
Purpose: To document the effect of the temporarily implanted nitinol device (iTind; Medi-Tate Ltd, Israel) on sexual function from a multicenter, randomized, single-blinded, sham-controlled trial. Materials and Methods: Men were randomized 2:1 between iTind and sham procedure arms. The iTind was placed for 5-7 days and an 18F Foley catheter was inserted and removed for the iTind and sham group, respectively. Patients were assessed at baseline, 3, and 12 months postoperatively using the Sexual Health Inventory for Men (SHIM) and International Index of Erectile Function (IIEF). Unblinding occurred at 3 months. Results: We studied 185 men with a mean age of 61.1 ± 6.5 years. There was no difference in SHIM or total IIEF between iTind and sham at 3 months or in the iTind arm at 12 months compared with baseline. Men in the iTind arm without erectile dysfunction at baseline showed an improvement in total IIEF score of +6.07 ± 21.17 points (p = 0.034) at 12 months, in addition to an improvement in ejaculatory function. SHIM scores remained unchanged in all groups, regardless of age, prostate volume, or baseline erectile function. Conclusion: No changes were observed in sexual and ejaculatory function of patients with iTind regardless of a man's age, prostate volume, and baseline sexual function. Clinicaltrials.gov: NCT02506465.
Keywords: lower urinary tract symptoms; men's health; minimally invasive surgical procedures; prostatic hyperplasia.
Conflict of interest statement
D.E. is on the medical advisory board. B.C. is a consultant for Medi-Tate Ltd., Olympus, Boston Scientific, and Medeon Bio. The other authors have no conflicts of interest.
Figures
References
-
- Cindolo L, Pirozzi L, Fanizza C, et al. . Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: Population-based cohort study. Eur Urol 2015;68(3):418–425; doi: 10.1016/j.eururo.2014.11.006 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
