Efficient Homology-Directed Repair with Circular Single-Stranded DNA Donors
- PMID: 36070530
- PMCID: PMC9595650
- DOI: 10.1089/crispr.2022.0058
Efficient Homology-Directed Repair with Circular Single-Stranded DNA Donors
Abstract
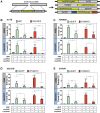
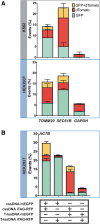
While genome editing has been revolutionized by the advent of CRISPR-based nucleases, difficulties in achieving efficient, nuclease-mediated, homology-directed repair (HDR) still limit many applications. Commonly used DNA donors such as plasmids suffer from low HDR efficiencies in many cell types, as well as integration at unintended sites. In contrast, single-stranded DNA (ssDNA) donors can produce efficient HDR with minimal off-target integration. In this study, we describe the use of ssDNA phage to efficiently and inexpensively produce long circular ssDNA (cssDNA) donors. These cssDNA donors serve as efficient HDR templates when used with Cas9 or Cas12a, with integration frequencies superior to linear ssDNA (lssDNA) donors. To evaluate the relative efficiencies of imprecise and precise repair for a suite of different Cas9 or Cas12a nucleases, we have developed a modified traffic light reporter (TLR) system (TLR-multi-Cas variant 1 [MCV1]) that permits side-by-side comparisons of different nuclease systems. We used this system to assess editing and HDR efficiencies of different nuclease platforms with distinct DNA donor types. We then extended the analysis of DNA donor types to evaluate efficiencies of fluorescent tag knockins at endogenous sites in HEK293T and K562 cells. Our results show that cssDNA templates produce efficient and robust insertion of reporter tags. Targeting efficiency is high, allowing production of biallelic integrants using cssDNA donors. cssDNA donors also outcompete lssDNA donors in template-driven repair at the target site. These data demonstrate that circular donors provide an efficient, cost-effective method to achieve knockins in mammalian cell lines.
Conflict of interest statement
No competing financial interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

