Enhancer Function and Evolutionary Roles of Human Accelerated Regions
- PMID: 36070559
- PMCID: PMC9712246
- DOI: 10.1146/annurev-genet-071819-103933
Enhancer Function and Evolutionary Roles of Human Accelerated Regions
Abstract
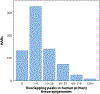
Human accelerated regions (HARs) are the fastest-evolving sequences in the human genome. When HARs were discovered in 2006, their function was mysterious due to scant annotation of the noncoding genome. Diverse technologies, from transgenic animals to machine learning, have consistently shown that HARs function as gene regulatory enhancers with significant enrichment in neurodevelopment. It is now possible to quantitatively measure the enhancer activity of thousands of HARs in parallel and model how each nucleotide contributes to gene expression. These strategies have revealed that many human HAR sequences function differently than their chimpanzee orthologs, though individual nucleotide changes in the same HAR may have opposite effects, consistent with compensatory substitutions. To fully evaluate the role of HARs in human evolution, it will be necessary to experimentally and computationally dissect them across more cell types and developmental stages.
Keywords: enhancer; epigenetics; evolution; human accelerated region; machine learning; reporter assay.
Figures


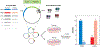

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

